In short, gas flow is a critical control parameter that directly dictates the chemical environment, reaction efficiency, and thermal stability within a split tube furnace. The rate and composition of the gas you introduce can either optimize your process for speed and yield or lead to unwanted side reactions, sample contamination, and even catastrophic failure of the furnace tube.
The core challenge is not simply to flow gas, but to achieve a precise balance. You must manage the gas flow to create the ideal chemical atmosphere while simultaneously avoiding dangerous thermal gradients that can damage the equipment.

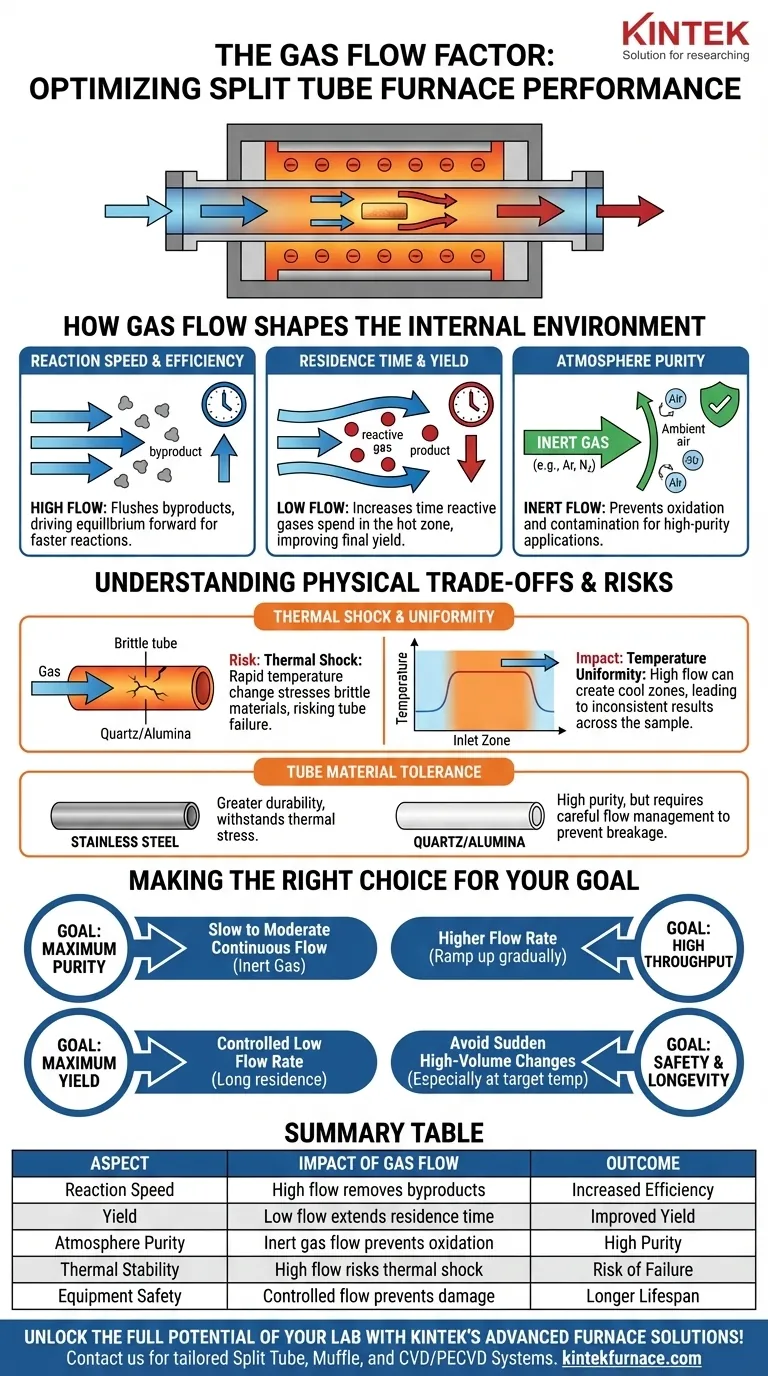
How Gas Flow Shapes the Internal Environment
A split tube furnace creates a precise thermal zone, but the gas you flow through the tube is what truly defines the processing environment. It is an active component, not a passive one.
Controlling Reaction Speed and Efficiency
A higher gas flow rate continuously flushes the reaction zone inside the tube. This is essential for removing gaseous byproducts that could otherwise slow down or inhibit the desired reaction.
By constantly removing these byproducts, you can drive the chemical equilibrium forward, often resulting in a faster and more efficient process.
Managing Residence Time for Optimal Yield
Conversely, a lower gas flow rate increases the residence time—the amount of time the reactive gases spend inside the hot zone.
For many chemical synthesis or material transformation processes, a longer residence time is necessary for the reaction to fully complete. This can significantly improve the final product yield.
Ensuring Atmosphere Purity
The composition of the gas is as important as its flow rate. Using inert gases like argon or nitrogen is standard practice for creating a clean, non-reactive environment.
This prevents unwanted oxidation or side reactions with ambient air, which is critical for high-purity applications like crystal growth or annealing sensitive materials.
Understanding the Physical Trade-offs and Risks
Choosing a gas flow rate is not just a chemical decision; it has direct physical consequences for the furnace components, particularly the process tube.
The Primary Risk: Thermal Shock
Introducing a cool gas at an excessive flow rate into a hot process tube creates a significant temperature difference. This thermal gradient induces stress in the tube material.
Brittle materials like quartz, often chosen for their high purity, are especially vulnerable to this thermal shock and can crack or shatter, compromising the experiment and the equipment.
The Impact on Temperature Uniformity
A high flow rate can also disrupt the furnace's temperature uniformity. The gas entering the tube absorbs heat, creating a cooler zone near the inlet compared to the center and outlet.
This lack of uniformity can lead to inconsistent results, especially in processes like annealing where every part of the sample must experience the same temperature profile.
The Role of Tube Material
The choice of tube material dictates its tolerance for aggressive gas flow.
Stainless steel tubes offer greater durability and can better withstand thermal stress, making them suitable for reactive atmospheres. In contrast, quartz or alumina tubes are chosen for purity and chemical resistance but require more careful management of gas flow to prevent breakage.
Making the Right Choice for Your Goal
Your optimal gas flow strategy depends entirely on the primary objective of your furnace process. Start by defining your goal, then adjust the gas flow to achieve it.
- If your primary focus is maximum purity: Use a slow to moderate, continuous flow of high-purity inert gas to gently purge contaminants without introducing significant thermal stress.
- If your primary focus is high throughput or byproduct removal: Use a higher flow rate, but be sure to ramp up the flow gradually as the furnace heats up to avoid shocking the tube.
- If your primary focus is maximum reaction yield: Use a carefully controlled, low flow rate to increase the residence time of reactants within the hot zone.
- If your primary focus is safety and equipment longevity: Always avoid sudden, high-volume changes in gas flow, especially when the furnace is at its target temperature.
Mastering your process begins with understanding that gas flow is your primary tool for controlling the environment inside the tube.
Summary Table:
| Aspect | Impact of Gas Flow |
|---|---|
| Reaction Speed | High flow removes byproducts, increasing efficiency |
| Yield | Low flow extends residence time, improving yield |
| Atmosphere Purity | Inert gas flow prevents oxidation and contamination |
| Thermal Stability | High flow risks thermal shock and uneven heating |
| Equipment Safety | Controlled flow prevents tube damage and failures |
Unlock the full potential of your lab with KINTEK's advanced high-temperature furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with tailored products like Split Tube Furnaces, Muffle Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures precise alignment with your unique experimental needs, enhancing efficiency and safety. Contact us today to discuss how we can optimize your processes and deliver reliable performance!
Visual Guide
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Multi Zone Laboratory Quartz Tube Furnace Tubular Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What safety and reliability features are incorporated into a vertical tube furnace? Ensuring Safe, Consistent High-Temp Processing
- How does a vertical tube furnace achieve precise temperature control? Unlock Superior Thermal Stability for Your Lab
- Why is a tube furnace utilized for the heat treatment of S/C composite cathode materials? Optimize Battery Stability
- What safety measures are essential when operating a lab tube furnace? A Guide to Preventing Accidents
- How is a Vertical Tube Furnace used for fuel dust ignition studies? Model Industrial Combustion with Precision



















