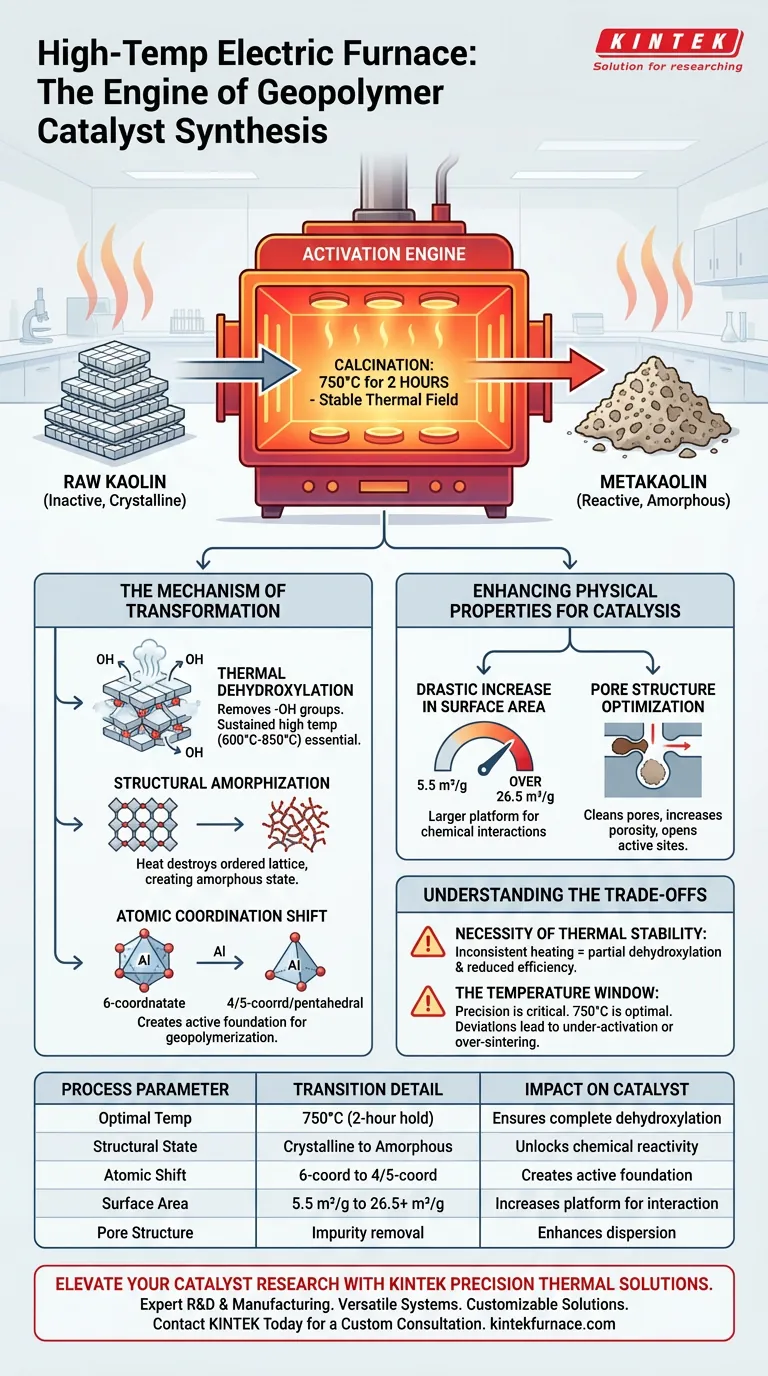
The high-temperature electric furnace acts as the primary activation engine in the synthesis of geopolymer catalysts. By subjecting raw kaolin to a stable thermal field—specifically calcination at 750°C for two hours—the furnace drives a critical process called dehydroxylation. This thermal treatment fundamentally converts chemically inert kaolin into metakaolin, an amorphous aluminosilicate with the high reactivity necessary for subsequent geopolymerization.
Core Takeaway The furnace does not simply dry the material; it triggers a molecular phase change. By collapsing the crystalline structure of kaolin through precise thermal control, the furnace "unlocks" the material’s potential, turning a passive mineral into an active chemical precursor required for effective catalysis.

The Mechanism of Transformation
Thermal Dehydroxylation
The primary function of the furnace is to facilitate dehydroxylation.
This is a chemical reaction where hydroxyl groups (-OH) are driven out of the kaolin structure.
Without the sustained high temperatures (typically between 600°C and 850°C) provided by the furnace, the material remains in a stable, unreactive state.
Structural Amorphization
Raw kaolin possesses a layered, ordered crystalline structure.
The furnace's heat destroys this lattice, causing the structure to collapse into an amorphous state.
This transition from ordered to disordered is the defining characteristic of metakaolin and is directly responsible for its ability to participate in geopolymer synthesis.
Atomic Coordination Shift
On an atomic level, the stable thermal field forces a shift in aluminum atoms.
They transition from a stable six-coordinate (octahedral) state to a highly unstable and reactive four- or five-coordinate (tetrahedral or pentahedral) state.
This atomic rearrangement creates the active foundation for the geopolymerization reaction.
Enhancing Physical Properties for Catalysis
Drastic Increase in Surface Area
The furnace treatment significantly alters the physical architecture of the material.
Calcination can expand the specific surface area from approximately 5.5 m²/g to over 26.5 m²/g.
This increase provides a much larger platform for chemical interactions, directly improving catalytic efficiency.
Pore Structure Optimization
The high heat thoroughly removes organic impurities, moisture, and volatile components clogged within the raw material.
This "cleaning" process opens up pore channels and increases porosity.
The result is a material with cleaner, more accessible active sites, facilitating better dispersion of active components during later stages.
Understanding the Trade-offs
The Necessity of Thermal Stability
A high-temperature electric furnace is chosen specifically for its ability to maintain a stable thermal field.
Inconsistent heating leads to partial dehydroxylation, leaving some kaolin inactive and reducing the overall efficiency of the catalyst.
The Temperature Window
Precision is critical; the process is not merely about reaching a high temperature, but reaching the right temperature.
While calcination can occur between 600°C and 850°C, the primary standard for this specific geopolymer application is 750°C.
Deviating significantly from this optimal window can result in either under-activated material or over-sintering, which would reduce reactivity.
Making the Right Choice for Your Goal
To ensure optimal catalyst preparation, align your furnace operations with your specific objectives:
- If your primary focus is maximum chemical reactivity: Ensure your furnace provides a stable hold at 750°C for two hours to guarantee complete dehydroxylation and the ideal atomic coordination shift.
- If your primary focus is maximizing surface area for impregnation: Prioritize the removal of organics and volatiles to clear pore channels, as this exposes the maximum number of active sites for metal ion interaction.
The electric furnace is not just a heating element; it is the tool that dictates the ultimate chemical potency of your catalyst.
Summary Table:
| Process Parameter | Transition Detail | Impact on Catalyst |
|---|---|---|
| Optimal Temp | 750°C (2-hour hold) | Ensures complete dehydroxylation |
| Structural State | Crystalline to Amorphous | Unlocks chemical reactivity |
| Atomic Shift | 6-coordinate to 4/5-coordinate | Creates active foundation for geopolymerization |
| Surface Area | 5.5 m²/g to 26.5+ m²/g | Increases platform for chemical interaction |
| Pore Structure | Impurity removal & opening channels | Enhances dispersion of active components |
Elevate Your Catalyst Research with Precision Thermal Solutions
High-performance geopolymer catalysts demand the absolute thermal stability and precise control that only expert-engineered systems can provide. KINTEK empowers researchers and manufacturers with advanced furnace technology designed to optimize your material transformation.
Our value to you:
- Expert R&D & Manufacturing: Backed by decades of expertise in high-temperature materials science.
- Versatile Systems: Choose from Muffle, Tube, Rotary, Vacuum, and CVD systems tailored for specific material phases.
- Customizable Solutions: Fully adjustable thermal profiles to meet your unique calcination and synthesis requirements.
Whether you are scaling production or refining molecular phase changes, KINTEK provides the reliability your lab requires.
Contact KINTEK Today for a Custom Consultation
Visual Guide
References
- Tuqa A. Jabar, Mayyadah S. Abed. Utilizing Kaolin-Based Geopolymer Catalysts for Improved Doura Vacuum Residue Cracking. DOI: 10.55699/ijogr.2024.0401.1061
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- How does a high-precision muffle furnace contribute to the evaluation of coatings? 1100°C Oxidation Test Insights
- What role does a Muffle furnace play in chemical reactions? Achieve Precise, Contamination-Free Thermal Processing
- Why is a laboratory muffle furnace essential for the activation of catalysts? Optimize Your Catalyst Performance
- What maintenance checks are required for a muffle furnace? Ensure Safety and Accuracy in Your Lab
- How does a vacuum furnace differ from a muffle furnace in terms of operation? Choose the Right Furnace for Your Lab
- Why is a laboratory furnace with an open quartz vessel utilized for CD2-type carbon dots? Precise Thermal Synthesis
- What is the role of an industrial box resistance furnace in the conversion of basic copper chloride to copper oxide?
- What role does an industrial-grade high-temperature muffle furnace play in the calcination of Barium Titanate powders?



















