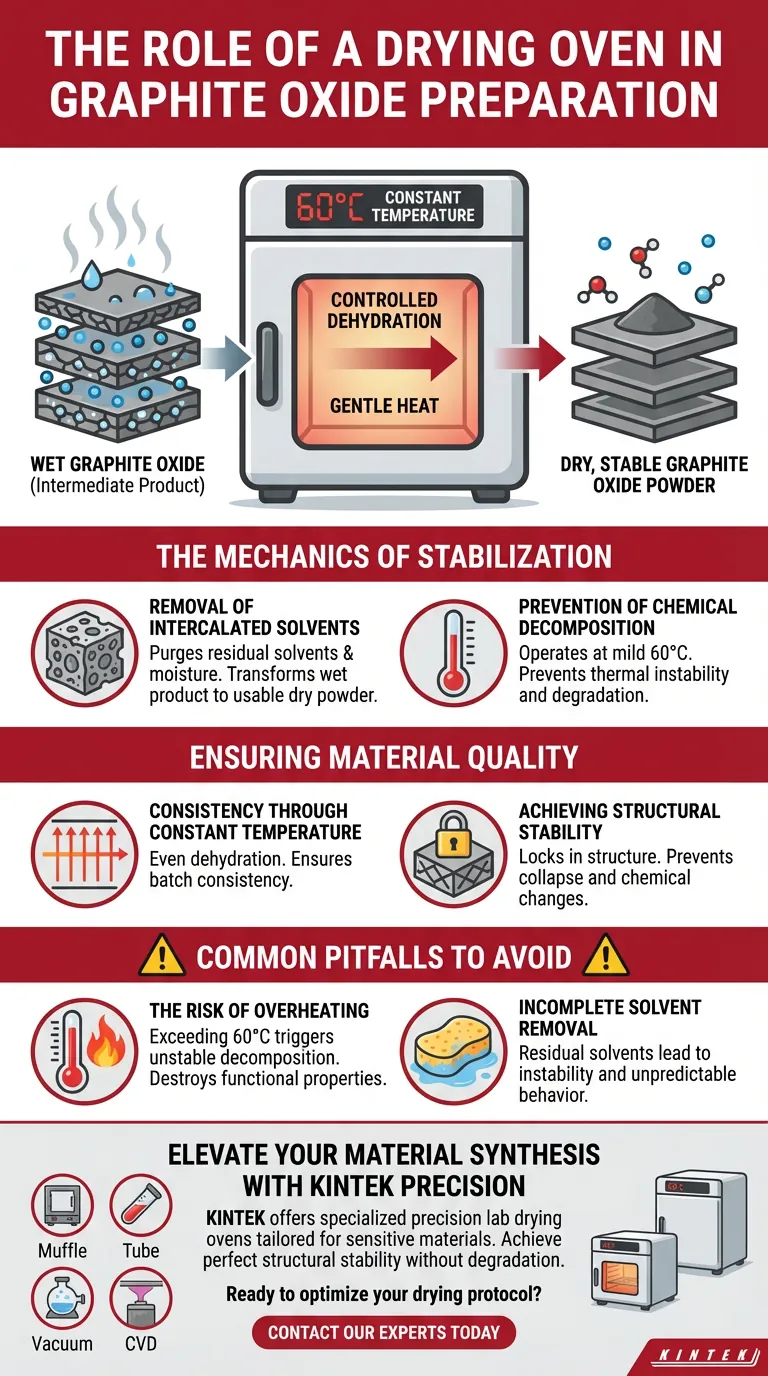
The primary role of a drying oven in graphite oxide preparation is to ensure controlled dehydration without compromising the material's chemical structure. Specifically, it utilizes a constant, mild temperature of 60°C to remove residual moisture and solvents trapped between the graphite layers, yielding a stable intermediate product.
The drying process is a delicate balance between purification and preservation. The oven eliminates volatile contaminants that would destabilize the powder, while strict temperature controls prevent the premature chemical decomposition that occurs at higher heat.
The Mechanics of Stabilization
Removal of Intercalated Solvents
The synthesis of graphite oxide involves various chemical solvents and water that become trapped within the material's layered structure.
An industrial-grade drying oven is essential to purge these residual solvents and moisture from between the graphite layers. This step transforms the wet, purified product into a usable, dry powder.
Prevention of Chemical Decomposition
Graphite oxide is thermally sensitive; it is chemically unstable at high temperatures.
The drying oven operates at a mild, constant temperature of 60°C. This specific thermal ceiling is critical because it provides enough energy to evaporate water but remains low enough to prevent the graphite oxide from decomposing or degrading.
Ensuring Material Quality
Consistency Through Constant Temperature
Fluctuations in heat can lead to uneven drying or localized degradation of the powder.
The drying oven provides a constant-temperature environment, ensuring that the entire batch of material dehydrates at the same rate. This uniformity is vital for producing a consistent, stable intermediate material ready for further processing or application.
Achieving Structural Stability
The ultimate goal of this phase is not just dryness, but stability.
By gently removing the volatile components, the oven "locks in" the structure of the graphite oxide. This results in a stable intermediate material, preventing the structural collapse or chemical changes that would render the powder ineffective for its intended use.
Common Pitfalls to Avoid
The Risk of Overheating
A common error in drying processes is increasing the temperature to speed up production.
In the context of graphite oxide, temperatures exceeding 60°C must be avoided. Excessive heat triggers unstable chemical decomposition, effectively destroying the functional properties of the graphite oxide before it can be used.
Incomplete Solvent Removal
Failing to dry the material for a sufficient duration or at the correct temperature results in retained moisture.
Residual solvents left between the layers can lead to instability over time. This compromises the material's shelf life and can cause unpredictable behaviors during subsequent chemical reactions or applications.
Making the Right Choice for Your Goal
To ensure the highest quality graphite oxide powder, your drying protocol must prioritize thermal control over speed.
- If your primary focus is Structural Integrity: Adhere strictly to the 60°C temperature limit to avoid thermally induced decomposition of the oxygen-containing groups.
- If your primary focus is Material Purity: Ensure the drying cycle is long enough to fully evacuate intercalated solvents from the graphite layers, as surface dryness alone is insufficient.
Precision in this final drying stage is the difference between a volatile, degraded byproduct and a stable, high-performance material.
Summary Table:
| Feature | Role in Graphite Oxide Preparation | Benefit to Material |
|---|---|---|
| Temperature Control | Constant 60°C thermal environment | Prevents premature chemical decomposition |
| Dehydration | Removal of intercalated solvents & water | Converts wet product into stable dry powder |
| Uniformity | Even heat distribution across layers | Ensures batch consistency and structural stability |
| Purification | Elimination of volatile contaminants | Prevents instability and improves shelf life |
Elevate Your Material Synthesis with KINTEK Precision
High-performance graphite oxide requires uncompromising thermal accuracy. Backed by expert R&D and manufacturing, KINTEK offers a specialized range of Muffle, Tube, Vacuum, and CVD systems, along with precision lab drying ovens tailored for sensitive materials. Whether you need standard or customizable high-temperature furnaces, our technology ensures your materials achieve perfect structural stability without degradation.
Ready to optimize your drying protocol? Contact our experts today to find the perfect solution for your lab!
Visual Guide
References
- Osman Eksik. Large-scale Production of Few-Layer Reduced Graphene Oxide by the Rapid Thermal Reduction of Graphene Oxide and Its Structural Characterization. DOI: 10.18596/jotcsa.1327988
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Dental Porcelain Sintering Furnace for Dental Laboratories
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What is the role of hydrate precursors in Mn3O4 nanosheet synthesis? Achieve Atomic-Level Dimensional Control
- How does controlled thermal treatment affect delta-MnO2? Optimize Porosity & Surface Area for Better Battery Performance
- How do CVT and hPLD process conditions for Nb1+xSe2 crystals differ? Exploring Equilibrium vs. Dynamic Growth
- What are the advantages of zirconia crowns? Achieve Durable, Aesthetic, and Biocompatible Dental Restorations
- What are the benefits of using electric actuators in this solution? Achieve Precision, Safety, and Efficiency in Automation
- How do microprocessor-controlled electric furnaces ensure the homogeneity of the Se80In5Te6Sb9 alloy?
- How does a multi speed furnace work? Achieve Ultimate Comfort & Efficiency
- Why is a fusion process using lithium metaborate necessary for the elemental analysis of S53P4 bioactive glass?



















