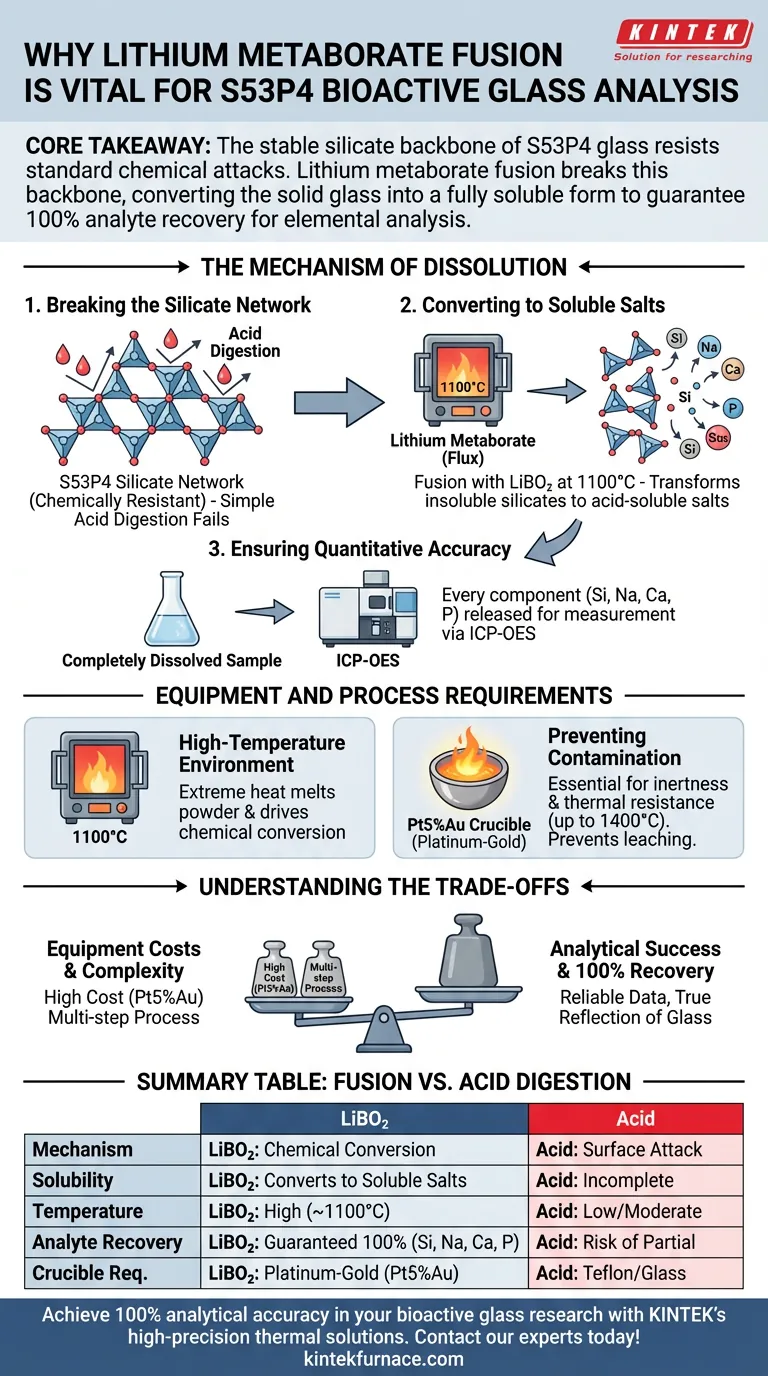
The necessity of lithium metaborate fusion lies in its ability to completely disassemble the chemically resistant structure of S53P4 bioactive glass. Standard acid digestion methods often fail to fully penetrate this material, but melting the glass powder with lithium metaborate at 1100°C converts the insoluble silicate network into soluble salts. This critical transformation ensures that silicon, sodium, calcium, and phosphorus are fully dissolved and available for accurate quantification via ICP-OES.
Core Takeaway: The stable silicate backbone of S53P4 glass resists standard chemical attacks. Lithium metaborate fusion breaks this backbone, converting the solid glass into a fully soluble form to guarantee 100% analyte recovery for elemental analysis.
The Mechanism of Dissolution
Breaking the Silicate Network
S53P4 bioactive glass is constructed around a robust, stable silicate network.
Because this structure is chemically resistant, simple acid digestion methods often result in incomplete dissolution.
Converting to Soluble Salts
Lithium metaborate acts as a powerful fluxing agent during the fusion process.
By reacting with the glass powder at high temperatures, it transforms the insoluble silicates into salts that dissolve readily in acid.
Ensuring Quantitative Accuracy
For techniques like ICP-OES (Inductively Coupled Plasma Optical Emission Spectroscopy), the sample must be in a completely liquid state.
Fusion ensures that every chemical component—specifically silicon, sodium, calcium, and phosphorus—is released from the solid phase for measurement.
Equipment and Process Requirements
High-Temperature Environment
To achieve the necessary reaction, the mixture must be heated to approximately 1100°C.
This extreme heat is required to melt the powder and drive the chemical conversion of the silicate network.
Preventing Sample Contamination
The molten glass mixture is highly corrosive, presenting a risk of dissolving the container holding it.
If the crucible material leaches into the sample, it compromises the purity and skews the elemental analysis.
The Role of Noble Metal Crucibles
To mitigate corrosion, a platinum-gold alloy (Pt5%Au) crucible is essential.
This alloy offers exceptional thermal resistance (up to 1400°C) and chemical inertness, ensuring the integrity of the bioactive glass composition is maintained.
Understanding the Trade-offs
Equipment Costs
While effective, this method requires significant capital investment in platinum-gold ware.
Using cheaper crucible alternatives is generally not an option due to the aggressive nature of the melt.
Process Complexity
This is a multi-step precursor process that adds time to the analytical workflow compared to simple digestion.
It requires precise temperature control and handling to ensure safety and accuracy.
Ensuring Analytical Success
To obtain reliable data on S53P4 bioactive glass, align your equipment and methods with your specific analytical goals.
- If your primary focus is total compositional accuracy: You must use lithium metaborate fusion to ensure the silicate network is fully solubilized.
- If your primary focus is sample purity: You must utilize Pt5%Au crucibles to prevent the corrosive melt from leaching contaminants into your solution.
Mastering the fusion process is the only way to guarantee that the data you read is a true reflection of the glass you created.
Summary Table:
| Feature | Lithium Metaborate Fusion | Standard Acid Digestion |
|---|---|---|
| Mechanism | Chemical conversion of silicate network | Surface-level chemical attack |
| Solubility | Converts insoluble silicates to soluble salts | Often results in incomplete dissolution |
| Temperature | High-temperature (approx. 1100°C) | Low to moderate heating |
| Analyte Recovery | Guaranteed 100% recovery for Si, Na, Ca, P | Risk of partial recovery/residue |
| Crucible Req. | Platinum-Gold (Pt5%Au) for inertness | Typically Teflon or glass vessels |
Achieve 100% analytical accuracy in your bioactive glass research with KINTEK’s high-precision thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers advanced Muffle, Tube, and Vacuum furnace systems, alongside specialized platinum-gold labware, all customizable to meet your specific fusion and sintering requirements. Don't compromise your data with incomplete dissolution—contact our technical experts today to equip your lab for success!
Visual Guide
References
- Jian Zheng, Julian R. Jones. Sol‐gel derived S53P4 bioactive glass. DOI: 10.1111/jace.70090
This article is also based on technical information from Kintek Furnace Knowledge Base .
People Also Ask
- How do chill rings specifically influence the temperature field distribution? Expert Insight into Crystal Casting
- What is the purpose of silver paste coating for BCZT ceramics? Ensuring Precision in Electrical Performance Testing
- What are the cost advantages of vacuum sublimation for magnesium purification? Eliminate Key Consumables.
- What effect does a laboratory hot plate have on 2D material heterostructures? Enhancing Interlayer Bonding Quality
- What is the purpose of using a furnace at 500 °C for catalyst support pretreatment? Optimize Purity and Performance
- Why is a laboratory vacuum evaporation system essential for the preparation of electrodes in high-performance solar cells?
- What role does an electric thermostatic drying oven play in the pre-treatment of Fe–Ni/AC catalysts? Essential Guide
- How does the addition of RhCl3 facilitate the synthesis of RhSeCl crystals? Unlock High-Quality Crystal Growth