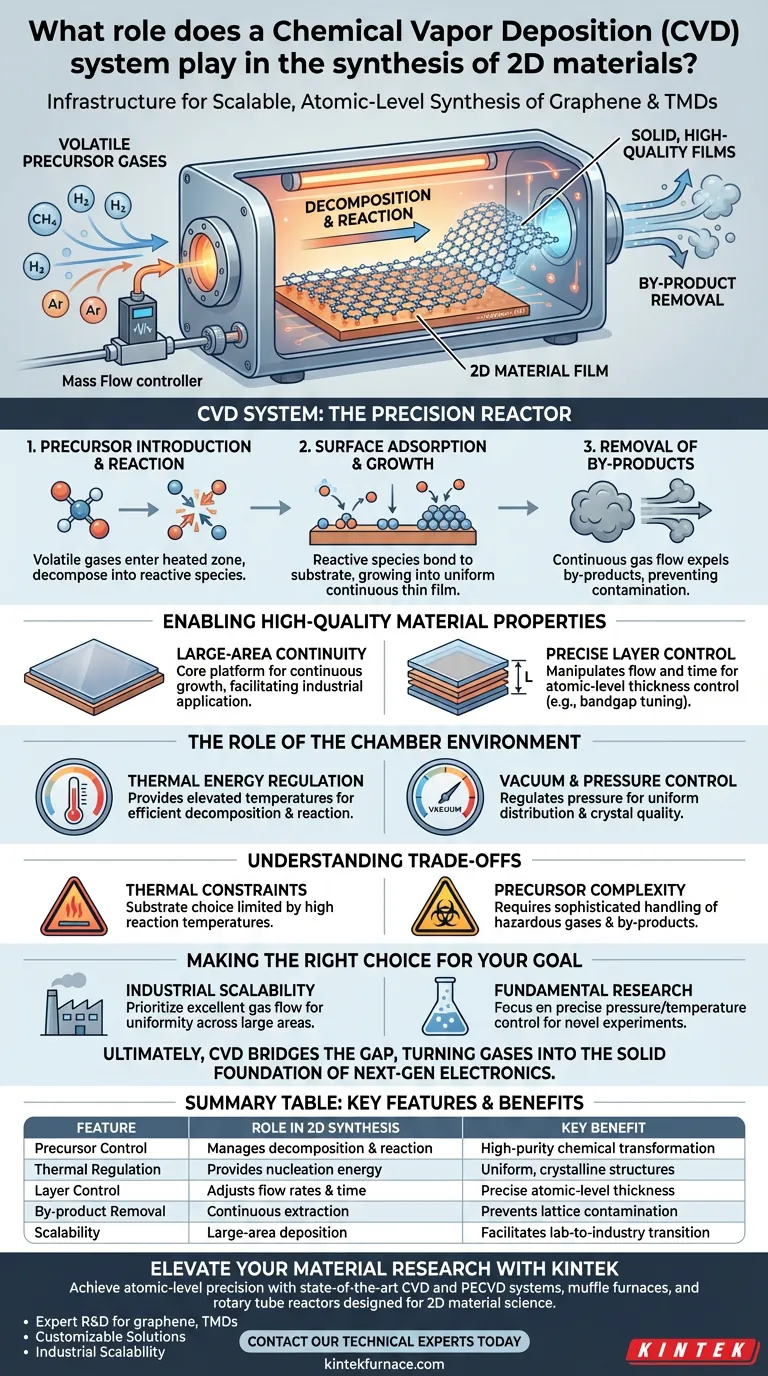
A Chemical Vapor Deposition (CVD) system serves as the primary infrastructure for the scalable synthesis of 2D materials. It functions by creating a high-temperature, precisely controlled environment where volatile precursor gases react chemically to deposit solid, high-quality films—such as graphene or transition metal dichalcogenides—onto a target substrate.
The CVD system is not merely a coating tool; it is a precision reactor that transforms gas-phase molecules into solid-state materials. It is the industry standard for achieving continuous, large-area growth while maintaining atomic-level control over layer thickness.

The Mechanism of Deposition
To understand the role of the CVD system, you must understand the transformation it orchestrates. The system manages the complex transition from a gaseous precursor to a solid 2D crystalline structure.
Precursor Introduction and Reaction
The process begins by introducing volatile gas precursors into the system's reaction chamber.
The system does not simply spray these gases; it facilitates a chemical reaction. As the gases enter the heated zone, they undergo decomposition or chemical reaction, often breaking down into reactive monomers or intermediate species.
Surface Adsorption and Growth
Once the precursors react in the gas phase or reach the substrate surface, they adsorb onto the material.
This is where the actual "synthesis" occurs. The reactive species bond to the substrate, nucleating and growing into a continuous thin film. This ensures the material creates a uniform coating on all exposed surfaces, rather than a line-of-sight deposition.
Removal of By-products
A critical function of the CVD system is waste management during synthesis.
As the solid film forms, volatile chemical by-products are generated. The system utilizes a continuous gas flow to expel these by-products from the chamber, preventing impurities from contaminating the newly formed 2D lattice.
Enabling High-Quality Material Properties
The CVD system is specifically valued in 2D material synthesis because it addresses the limitations of other methods (like mechanical exfoliation).
Achieving Large-Area Continuity
The primary reference highlights that CVD is the core platform for continuous growth.
Unlike methods that produce small, isolated flakes, a CVD system can synthesize materials over large surface areas. This is essential for moving 2D materials from the lab to industrial applications.
Precise Layer Control
The system allows for strict control over the thickness of the deposited material.
By manipulating the flow rate of precursors and the reaction time, the system can achieve "layer-controlled" synthesis. This allows researchers to target specific material properties that depend on the number of atomic layers (e.g., bandgap changes in semiconductors).
The Role of the Chamber Environment
The "hardware" role of the CVD system is to maintain rigorous environmental parameters.
Thermal Energy Regulation
The system provides the elevated temperatures necessary to drive the chemical reactions.
Whether decomposing a dimer or activating a surface reaction, the thermal environment is the catalyst. The system maintains this heat to ensure the reaction proceeds efficiently and uniformly across the substrate.
Vacuum and Pressure Control
Most CVD processes operate within a specific pressure range, from atmospheric to high vacuum.
The system regulates this pressure to control the mean free path of the gas molecules. This ensures uniform distribution of the precursor gas and helps determine the crystal quality of the final film.
Understanding the Trade-offs
While CVD is the standard for high-quality 2D synthesis, it introduces specific complexities that must be managed.
Thermal Constraints on Substrates
Because the process relies on high temperatures to decompose precursors, the choice of substrate is limited. You cannot easily deposit onto temperature-sensitive materials (like certain plastics) without degrading the target object.
Precursor Complexity
The process relies on volatile and often hazardous chemical precursors. Handling these requires sophisticated safety systems and scrubbers to manage the toxic by-products exhausted from the chamber.
Making the Right Choice for Your Goal
The CVD system is a versatile tool, but its application depends on your specific end-goal for the 2D material.
- If your primary focus is Industrial Scalability: Prioritize a system with excellent gas flow dynamics to ensure uniformity across large-area substrates.
- If your primary focus is Fundamental Research: Focus on a system with precise pressure and temperature controls to experiment with layer thickness and novel material compositions.
Ultimately, the CVD system bridges the gap between theoretical chemistry and physical application, turning volatile gases into the solid foundation of next-generation electronics.
Summary Table:
| Feature | Role in 2D Synthesis | Key Benefit |
|---|---|---|
| Precursor Control | Manages gas decomposition & reaction | Ensures high-purity chemical transformation |
| Thermal Regulation | Provides energy for film nucleation | Achieves uniform, crystalline structures |
| Layer Control | Adjusts flow rates and reaction time | Enables precise atomic-level thickness |
| By-product Removal | Continuous gas flow extraction | Prevents contamination of the 2D lattice |
| Scalability | Large-area substrate deposition | Facilitates transition from lab to industry |
Elevate Your Material Research with KINTEK
Ready to achieve atomic-level precision in your synthesis? KINTEK provides state-of-the-art CVD and PECVD systems, muffle furnaces, and rotary tube reactors designed specifically for the rigorous demands of 2D material science.
Why choose KINTEK?
- Expert R&D: Systems engineered for graphene, TMDs, and carbon nanotubes.
- Customizable Solutions: Tailored vacuum and thermal configurations for your unique research needs.
- Industrial Scalability: High-performance manufacturing to bridge the gap from lab to production.
Take the next step in next-generation electronics—contact our technical experts today to find the perfect high-temperature solution for your laboratory.
Visual Guide
References
- Yixin Chen, Nan Zhang. Advance in additive manufacturing of 2D materials at the atomic and close-to-atomic scale. DOI: 10.1038/s41699-024-00456-x
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- How is CVD classified based on physical characteristics of vapor? Explore AACVD and DLICVD Methods
- How does process complexity compare between PVD and CVD? Uncover Key Differences for Your Lab
- What are the main disadvantages of Chemical Vapor Deposition (CVD)? High Costs, Complex Control, and Safety Risks
- What are the advantages of chemical vapor deposition? Achieve Superior, Conformal Films for Complex 3D Structures
- What is the pressure range for CVD furnaces? Optimize Thin Film Deposition for Your Lab
- What types of materials can be deposited using CVD in microfabrication? Explore Versatile Thin Films for Your Devices
- When should CVD be chosen over PVD for thin-film deposition? Unlock Superior Conformality for Complex Shapes
- What are the different types of CVD systems and their applications? Choose the Right CVD for Your Lab Needs



















