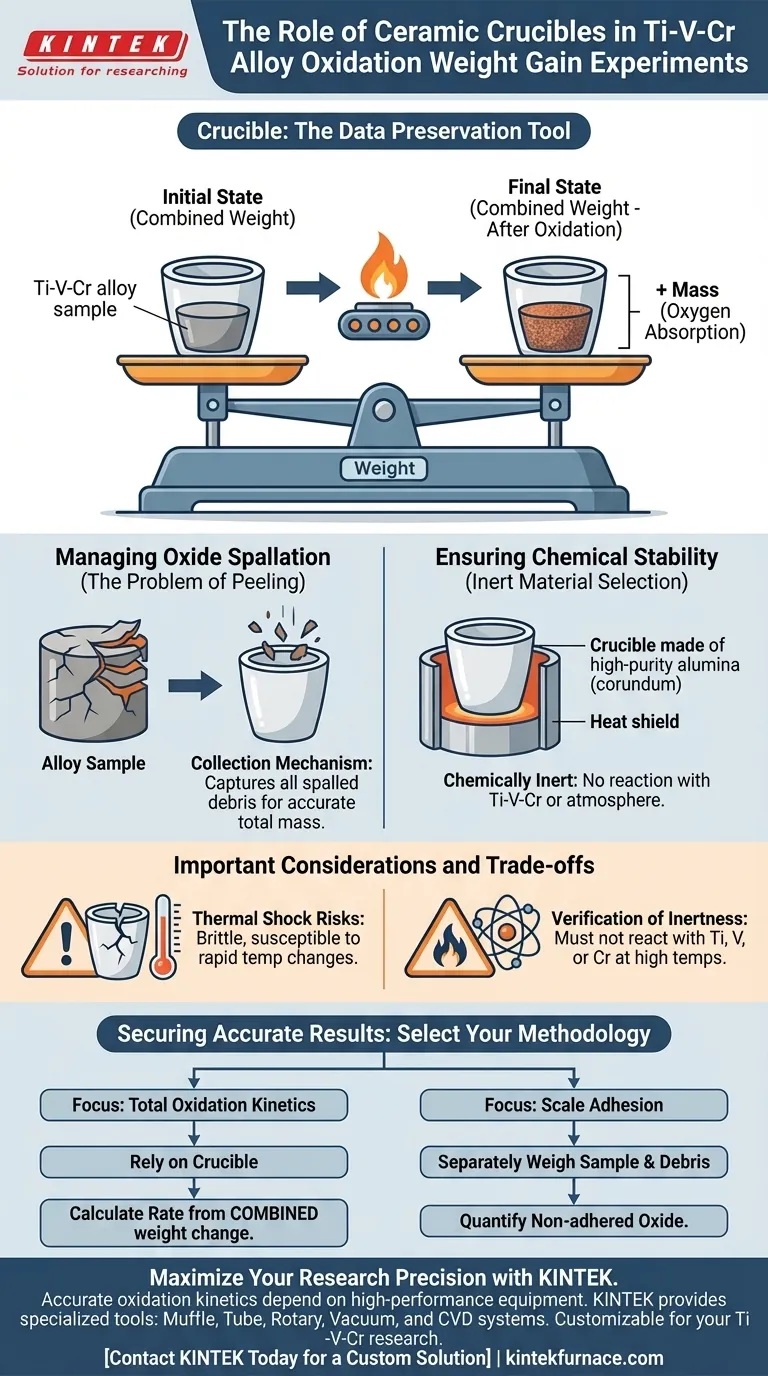
In oxidation weight gain experiments for Ti-V-Cr alloys, the ceramic crucible acts as a chemically inert containment vessel that preserves the integrity of the total mass balance. It allows researchers to determine oxidation rates by measuring the combined weight of the vessel and the specimen, ensuring that no mass is lost even if the oxide scale detaches from the metal.
The crucible is not merely a holder; it is a data preservation tool. Its primary function is to collect any oxide scale that spalls (peels) off the alloy, ensuring the final weight measurement accurately reflects the total oxygen absorbed by the system.
The Principles of Mass Gain Measurement
The "Combined Weight" Technique
In these experiments, accuracy relies on tracking the total system mass. Researchers do not weigh the Ti-V-Cr specimen in isolation after heating.
Instead, they measure the combined weight of the ceramic crucible and the specimen together. This baseline is established before the experiment begins and is compared against the total weight after the oxidation process.
Capturing Minute Changes
Oxidation involves the absorption of oxygen atoms into the metal lattice or the formation of surface layers. This results in a mass increase.
Using a crucible allows analytical balances to capture these often minute mass changes without the interference of handling the specimen directly, which could disturb the fragile oxide layer.
Managing Oxide Spallation
The Problem of Peeling
Ti-V-Cr alloys, like many high-temperature metals, develop an oxide scale when exposed to heat.
Under thermal stress or due to specific growth kinetics, this oxide layer may crack, peel, or spall off the surface of the sample.
The Collection Mechanism
If a sample were suspended or placed on a flat tray without containment, spalled oxide debris would fall away and be lost.
The ceramic crucible solves this by collecting all falling debris. Because the debris is trapped within the vessel, its mass is included in the final weighing, guaranteeing that the calculated weight gain represents the true extent of oxidation.
Ensuring Chemical Stability
Inert Material Selection
The crucible must be manufactured from materials that possess high chemical stability, such as high-purity corundum (alumina).
Preventing Cross-Reactions
The crucible must remain inert at high temperatures. It must not react with the Ti-V-Cr alloy or the oxidizing atmosphere.
Any chemical interaction between the crucible and the specimen would alter the mass artificially, corrupting the experimental data.
Important Considerations and Trade-offs
Verification of Inertness
While high-purity ceramics are generally stable, Titanium is highly reactive at elevated temperatures.
One must verify that the specific ceramic composition chosen does not undergo solid-state reactions with the Titanium, Vanadium, or Chromium content at the target experimental temperature.
Thermal Shock Risks
Ceramic crucibles are brittle and susceptible to thermal shock.
Rapid heating or cooling cycles can cause the crucible to crack. A cracked crucible may lose mass (chips falling off) or allow oxide debris to escape, invalidating the weight gain data.
Securing Accurate Results
To ensure the validity of your Ti-V-Cr oxidation data, select your methodology based on your specific analytical goals:
- If your primary focus is Total Oxidation Kinetics: Rely on the crucible to capture all spalled mass; calculate rates based strictly on the combined weight change of the crucible and sample.
- If your primary focus is Scale Adhesion: Separately weigh the sample and the debris collected in the crucible to quantify exactly how much of the oxide layer failed to adhere to the substrate.
By treating the crucible as an integral part of the measurement system, you ensure that physical degradation of the sample does not lead to a loss of data.
Summary Table:
| Feature | Function in Oxidation Experiments | Impact on Data Accuracy |
|---|---|---|
| Containment | Captures spalled oxide debris/peeling layers | Prevents underestimation of oxygen gain |
| Inertness | Resists reaction with Ti-V-Cr at high temps | Ensures weight change is purely from oxidation |
| Combined Mass | Measured together with specimen (Vessel + Alloy) | Enables precise tracking of total system mass |
| Material Purity | Typically high-purity alumina (corundum) | Minimizes cross-contamination during heating |
Maximize Your Research Precision with KINTEK
Accurate oxidation kinetics depend on high-performance laboratory equipment. KINTEK provides the specialized tools needed for demanding materials science, from high-purity ceramic crucibles to advanced heating systems.
Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable for your unique Ti-V-Cr alloy research or industrial needs. Ensure your experiments are supported by the best in high-temperature technology.
Contact KINTEK Today for a Custom Solution
Visual Guide
References
- Yuanzhi Sun, Liangju He. Prediction of oxidation resistance of Ti-V-Cr burn resistant titanium alloy based on machine learning. DOI: 10.1038/s41529-025-00553-2
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What functions does the hot pressing mold perform? Key Roles in Al3Ti/Al Composite Powder Metallurgy
- What is the function of a graphite plate in microwave cladding? Ensure Purity & Thermal Uniformity for HEA Synthesis
- What is the role of providing a uniform heating environment? Achieve Perfect Deep Eutectic Solvent Formation
- Are alumina ceramic furnace tubes suitable for high-pressure applications? Discover Key Factors for Safe Use
- What is the role of a high-temperature ceramic boat during phosphidation? Ensure Pure and Stable Chemical Synthesis
- Why are stainless steel tubes used during the cooling and heat treatment stages of Ti–Nb–Si alloys? Key Cooling Insights
- Why use high-performance insulation bricks in radiant tube simulations? Ensure precision and industrial accuracy.
- Why is a high-purity alumina (Al2O3) crucible required for the melting of nickel-based superalloys?
















