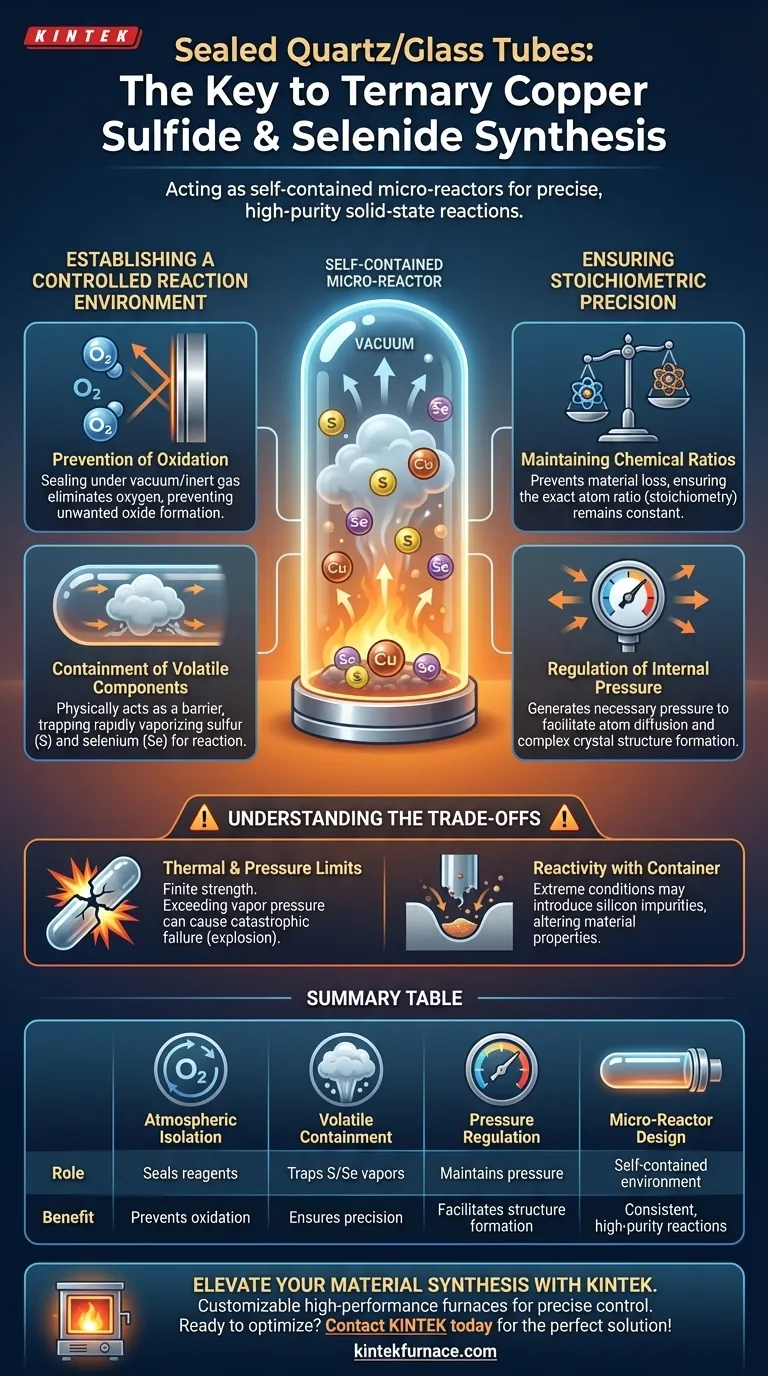
Sealed quartz or glass tubes function as self-contained micro-reactors that are critical for the successful synthesis of ternary copper sulfides and selenides. By encapsulating reagents under vacuum or inert gas, this technique isolates the reaction from atmospheric interference while containing volatile elements to ensure the final chemical composition is precise.
The core value of this method is the creation of a strictly controlled closed system. It simultaneously prevents the degradation of precursors through oxidation and traps volatile components that would otherwise escape, ensuring the final material matches the intended chemical formula.

Establishing a Controlled Reaction Environment
To understand why this method is standard, you must look at the specific vulnerabilities of copper chalcogenides during high-temperature synthesis.
Prevention of Oxidation
At the elevated temperatures required for solid-state reactions, raw materials become highly reactive.
Without a barrier, atmospheric oxygen interacts with the reagents, leading to the formation of unwanted oxides rather than the desired sulfide or selenide phases.
Sealing the tube under vacuum or inert gas effectively eliminates oxygen, ensuring the reaction proceeds only between the intended elements.
Containment of Volatile Components
Sulfur and selenium are volatile elements that vaporize rapidly at reaction temperatures.
In an open or unsealed system, these components would escape as gas, leaving the reaction mixture deficient in essential building blocks.
The sealed tube acts as a physical barrier, trapping these vapors within the reaction zone so they remain available to react with the copper and other metals.
Ensuring Stoichiometric Precision
The success of ternary synthesis depends entirely on the ratio of atoms in the final structure.
Maintaining Chemical Ratios
Because the sealed system prevents material loss, the stoichiometry (the exact ratio of elements) remains constant throughout the heating process.
If volatile selenium or sulfur were allowed to escape, the final product would likely be a mixture of impurities or a completely different phase than intended.
Regulation of Internal Pressure
As the synthesis proceeds, the vaporization of components generates internal pressure.
The sealed quartz or glass tube maintains these necessary pressure conditions, which can drive the kinetics of the solid-state reaction.
This pressurized environment facilitates the diffusion of atoms, allowing the formation of the complex crystal structures typical of ternary compounds.
Understanding the Trade-offs
While effective, using sealed tubes introduces specific physical limitations and risks that must be managed.
Thermal and Pressure Limits
Glass and quartz have finite strength limits regarding temperature and internal pressure.
If the vapor pressure generated by the sulfur or selenium exceeds the tube's tensile strength, the vessel will fail catastrophically (explode).
Reactivity with the Container
While quartz is generally inert, there are extreme conditions or specific precursors that may react with the tube walls over time.
This can introduce silicon impurities into the sample, subtly altering the properties of the final material.
Making the Right Choice for Your Goal
When designing your synthesis protocol, the sealing method should align with your specific experimental requirements.
- If your primary focus is precise stoichiometry: Ensure a high-quality vacuum seal to prevent even trace amounts of volatile loss or oxidation.
- If your primary focus is safety during high-pressure reactions: Calculate the free volume of the tube carefully to ensure the vapor pressure generated by the chalcogens does not exceed the quartz's burst limit.
By controlling the atmosphere and pressure, you turn a chaotic chemical mixture into a predictable, high-purity material.
Summary Table:
| Feature | Role in Synthesis | Benefit to Final Material |
|---|---|---|
| Atmospheric Isolation | Seals reagents under vacuum or inert gas | Prevents oxidation and formation of unwanted oxides |
| Volatile Containment | Traps sulfur and selenium vapors | Ensures stoichiometric precision and correct chemical formula |
| Pressure Regulation | Maintains internal vapor pressure | Facilitates atom diffusion and complex crystal structure formation |
| Micro-Reactor Design | Creates a self-contained environment | Allows for consistent, high-purity solid-state reactions |
Elevate Your Material Synthesis with KINTEK
Precision in synthesizing ternary copper sulfides and selenides requires reliable thermal equipment. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to your laboratory's unique high-temperature needs. Whether you require precise atmosphere control or robust pressure management, our lab furnaces provide the stability your research demands.
Ready to optimize your synthesis process? Contact KINTEK today to find the perfect furnace solution for your lab!
Visual Guide
References
- С.А. Новиков, Vladislav V. Klepov. Structural evolution and bonding features of electron deficient copper chalcogenides. DOI: 10.1039/d5ce00479a
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What are the heating zone options for Tube Furnaces? Choose Single or Multi-Zone for Optimal Thermal Control
- Why is a specific nitrogen flow rate necessary within a tube furnace during the carbonization of PVDF?
- What are the different types of tube furnaces available? Find the Perfect Fit for Your Lab's Needs
- What is the operational principle of a 70mm tube furnace? Master Precise Heat and Atmosphere Control
- How are rotary tube furnaces used in the mining and metallurgy industry? Boost Efficiency in Metal Processing
- What are the typical working temperature ranges for lab tube furnaces? Find the Right Furnace for Your Process
- What are the advantages of vertical tube furnaces? Achieve Precision and Efficiency in Your Lab
- What factors contribute to the strong process performance of vacuum tube furnaces? Unlock Precision and Purity in Heat Treatment



















