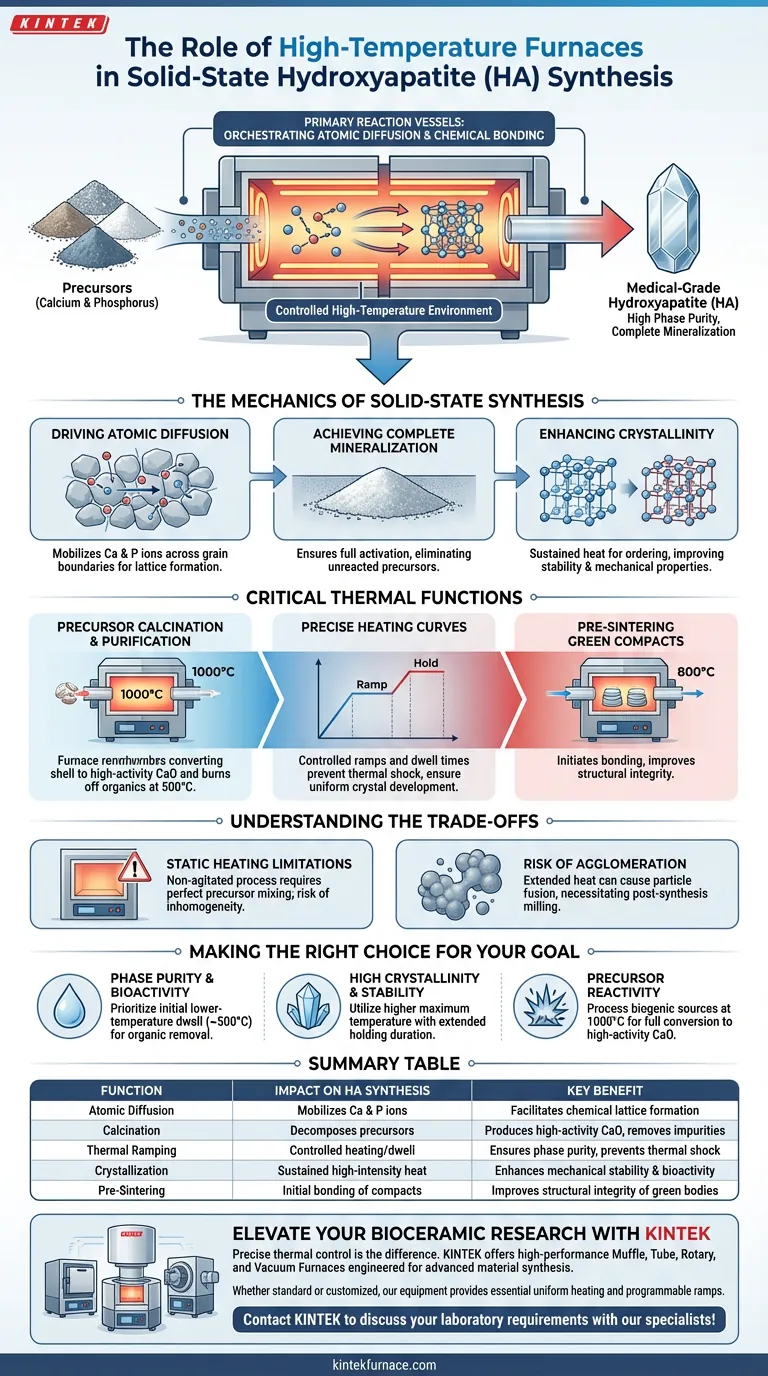
High-temperature muffle and tube furnaces serve as the primary reaction vessels that drive the solid-state synthesis of hydroxyapatite (HA). These devices provide the continuous, controlled high-temperature environment required to overcome the kinetic barriers of solid-phase reactions. By enabling precise regulation of heating curves and dwell times, they facilitate the atomic diffusion and chemical bonding between calcium and phosphorus precursors, directly dictating the final material's purity and crystalline structure.
Core Takeaway These furnaces do not merely heat materials; they orchestrate the atomic diffusion process required to transform raw precursors into stable bioceramics. By maintaining strict thermal profiles, they ensure complete mineralization and high phase purity, which are the defining characteristics of medical-grade hydroxyapatite.

The Mechanics of Solid-State Synthesis
Driving Atomic Diffusion
In solid-state synthesis, the reactants are solids, meaning atoms must physically move (diffuse) across grain boundaries to react.
High-temperature furnaces provide the necessary thermal energy to mobilize calcium and phosphorus ions. This facilitates the chemical interaction required to form the hydroxyapatite crystal lattice.
Achieving Complete Mineralization
Reaction completeness is critical for biocompatibility.
The furnace ensures that the entire powder bed reaches the specific activation energy required for the reaction. This eliminates unreacted precursors, ensuring the final product is fully mineralized hydroxyapatite rather than a mixture of raw salts.
Enhancing Crystallinity
The duration and intensity of the heat treatment directly impact the ordering of the atomic structure.
Sustained high temperatures allow the hydroxyapatite crystals to grow and perfect their structure. This results in high crystallinity, which correlates with better thermal stability and improved mechanical properties in the final application.
Critical Thermal Functions
Precursor Calcination and Purification
Before the final synthesis, these furnaces are often used to prepare the raw materials.
For biogenic sources (like eggshells or snail shells), furnaces operating around 1000°C thermally decompose calcium carbonate into high-activity calcium oxide (CaO). Simultaneously, temperatures around 500°C effectively burn off volatile organic impurities, ensuring a chemically pure starting material.
Precise Heating Curves
The quality of the final powder depends on how heat is applied, not just the maximum temperature reached.
Tube and muffle furnaces allow for programmable heating ramps (rate of temperature increase) and holding times. This prevents thermal shock and allows for the gradual, uniform development of the crystal phase.
Pre-Sintering Green Compacts
In some processing routes, the powder is cold-pressed into "green" compacts before final firing.
The furnace heats these compacts to intermediate temperatures (e.g., 800°C). This preliminary step initiates bonding between particles, improving the structural integrity of the compact before it undergoes final densification.
Understanding the Trade-offs
Static Heating Limitations
Unlike fluid bed reactors or rotary kilns, muffle furnaces provide a static heating environment.
Because the powder is not agitated during heating, the initial mixing of precursors must be perfect. Any inhomogeneity in the raw mixture will result in localized impurities, as the furnace cannot mechanically homogenize the batch during the reaction.
Risk of Agglomeration
High temperatures drive reaction, but they also drive sintering.
Extended dwell times or excessive temperatures can cause individual HA particles to fuse together (agglomerate) into hard clumps. This frequently necessitates a post-synthesis milling step to return the material to a fine, usable powder.
Making the Right Choice for Your Goal
To maximize the effectiveness of your furnace operations, align your thermal profile with your specific material requirements:
- If your primary focus is phase purity and bioactivity: Prioritize a lower-temperature dwell (approx. 500°C) initially to ensure the complete removal of volatile organics before ramping up for synthesis.
- If your primary focus is high crystallinity and thermal stability: utilize a higher maximum temperature with an extended holding duration to allow the crystal lattice to fully order and stabilize.
- If your primary focus is precursor reactivity: Process biogenic calcium sources at 1000°C to ensure full conversion to high-activity Calcium Oxide (CaO) prior to mixing with phosphorus sources.
Precise thermal management transforms a simple mixture of chemical salts into a sophisticated, medical-grade bioceramic.
Summary Table:
| Function | Impact on Hydroxyapatite (HA) Synthesis | Key Benefit |
|---|---|---|
| Atomic Diffusion | Mobilizes Ca and P ions across grain boundaries | Facilitates chemical lattice formation |
| Calcination | Decomposes biogenic precursors (e.g., shells) | Produces high-activity CaO & removes impurities |
| Thermal Ramping | Controlled heating and dwell cycles | Ensures phase purity and prevents thermal shock |
| Crystallization | Sustained high-intensity heat treatment | Enhances mechanical stability and bioactivity |
| Pre-Sintering | Initial bonding of cold-pressed compacts | Improves structural integrity of green bodies |
Elevate Your Bioceramic Research with KINTEK
Precise thermal control is the difference between raw precursors and medical-grade hydroxyapatite. Backed by expert R&D and world-class manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, and Vacuum furnaces specifically engineered for advanced material synthesis. Whether you need a standard setup or a fully customized CVD system for unique bioceramic needs, our equipment provides the uniform heating and programmable ramps essential for high-purity results.
Ready to optimize your solid-state synthesis? Contact KINTEK today to discuss your laboratory requirements with our specialists!
Visual Guide
References
- Liviu Duta, Valentina Grumezescu. The Effect of Doping on the Electrical and Dielectric Properties of Hydroxyapatite for Medical Applications: From Powders to Thin Films. DOI: 10.3390/ma17030640
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What role does a dual porcelain boat layout play within a tube furnace? Enhance Ni-N-C Selenization with Spatial Control
- How does a high-temperature tube furnace facilitate sulfur melt-diffusion? Precision Heating for PCFC/S Cathodes
- What are the key considerations for placing a multi zone tube furnace? Ensure Safety, Accuracy, and Longevity
- Why is a high-temperature tube furnace required for Ti3AuC2 annealing? Achieve Perfect Atomic Exchange
- What environmental conditions must a high-temperature tube furnace provide for MAX phase sintering? Expert Guidelines
- What is the role of horizontal furnaces in battery manufacturing? Achieve Precision Thermal Processing for Superior Battery Performance
- Why is a quartz tube fixed-bed reactor ideal for VOC/Hydrogen combustion? Unlock High-Temp Precision & Stability
- Why is a high-purity argon supply system and a flow meter integrated into a tube furnace? Optimize Pyrolysis Quality



















