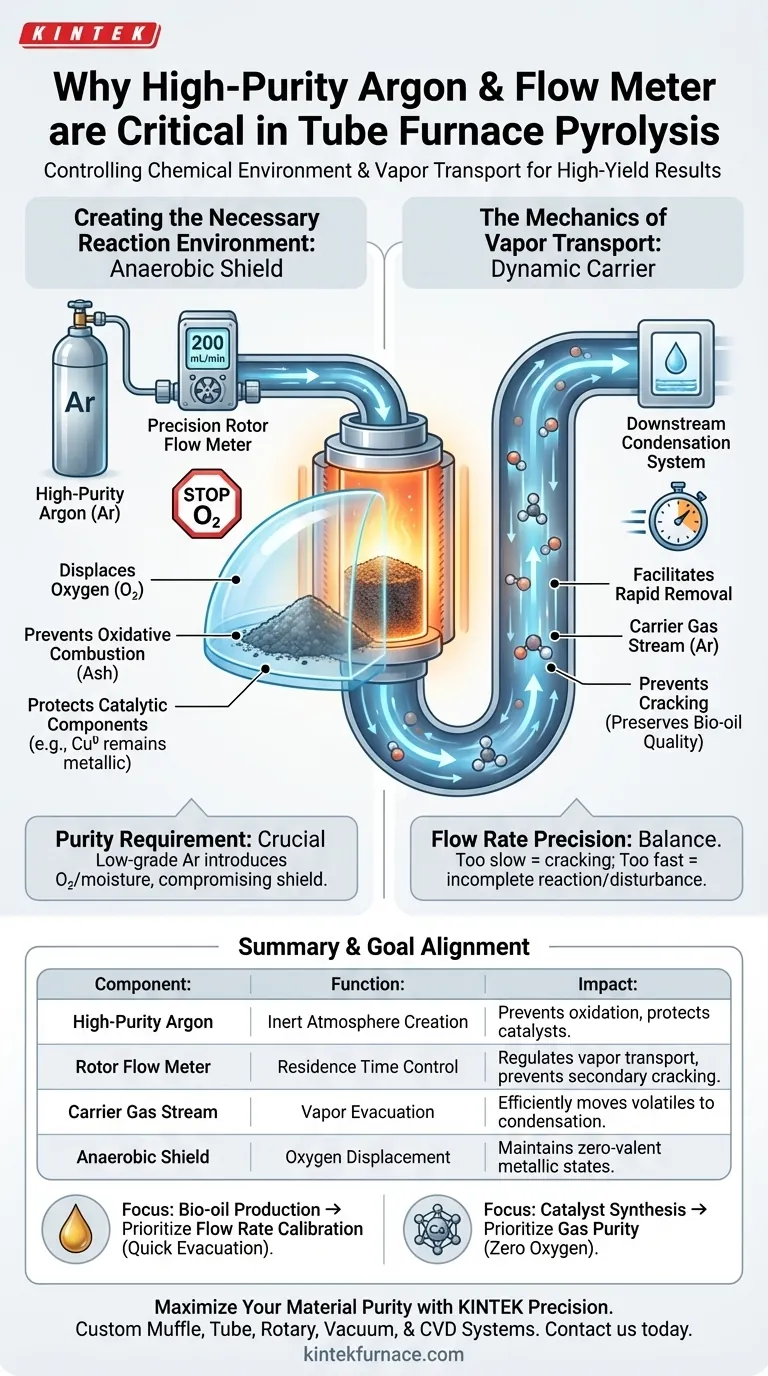
A high-purity argon supply system coupled with a precision flow meter is the critical infrastructure for controlling the chemical environment within a tube furnace. By regulating the gas flow—typically to a specific rate such as 200 mL/min—this setup systematically displaces oxygen to prevent combustion while simultaneously acting as a transport vehicle. This dual function ensures that sensitive materials do not oxidize and that volatile vapors are evacuated before they can degrade.
Pyrolysis relies on a delicate balance: the argon supply creates an anaerobic shield to prevent material destruction, while the flow meter dictates the speed at which products are removed to preserve their chemical integrity.

Creating the Necessary Reaction Environment
Establishing an Anaerobic State
The fundamental requirement of pyrolysis is thermal decomposition in the absence of oxygen.
The argon supply acts as an inert "blanket." It purges the reaction chamber, effectively removing air to ensure the environment is strictly anaerobic.
Preventing Oxidative Combustion
Without this inert atmosphere, the carbon substrate within the furnace would simply burn.
The introduction of high-purity argon prevents the carbon from undergoing oxidative combustion, allowing it to transform structurally rather than turning to ash.
Protecting Catalytic Components
For setups involving metallic catalysts, the absence of oxygen is non-negotiable.
Specific materials, such as copper nanoparticles, must remain in their zero-valent metallic state (Cu0) to function correctly. The argon shield prevents these particles from oxidizing into copper oxide, preserving their active properties for applications like iodine removal.
The Mechanics of Vapor Transport
Facilitating Rapid Removal
The argon is not merely a static atmosphere; it is a dynamic carrier gas.
Regulated by the rotor flow meter, the gas stream physically pushes volatile vapors out of the high-temperature zone. It transports them efficiently toward the downstream condensation system.
Preventing Secondary Cracking
The speed of transport is directly tied to product quality.
If volatile vapors remain in the heat zone for too long, they undergo "secondary cracking," breaking down into smaller, less desirable molecules. The carrier gas ensures these vapors are evacuated quickly, which is critical for maintaining the quality of products like bio-oil.
Understanding the Trade-offs
The Importance of Flow Rate Precision
The rotor flow meter is not an optional accessory; it is a control variable.
A rate of 200 mL/min is often calibrated to balance residence time. If the flow is too slow, secondary cracking ruins the bio-oil; if it is too fast, it may prevent necessary reactions or disturb the sample.
Material Purity Requirements
The effectiveness of the system relies entirely on the purity of the argon.
Using low-grade argon introduces trace oxygen or moisture. This compromises the anaerobic environment, leading to the very oxidation or catalyst degradation the system was designed to prevent.
Making the Right Choice for Your Goal
To optimize your tube furnace setup, align your gas control strategy with your specific objective:
- If your primary focus is Bio-oil Production: Prioritize flow rate calibration to ensure volatile vapors are evacuated immediately to prevent secondary cracking.
- If your primary focus is Catalyst Synthesis (e.g., Copper): Prioritize gas purity to ensure zero oxygen is present, maintaining metals in their active, zero-valent state.
Ultimately, the quality of your pyrolysis output is dictated by how effectively you control the atmosphere and the residence time of the vapors.
Summary Table:
| Component | Primary Function | Impact on Pyrolysis |
|---|---|---|
| High-Purity Argon | Inert Atmosphere Creation | Prevents oxidative combustion and protects catalysts |
| Rotor Flow Meter | Residence Time Control | Regulates vapor transport speed to prevent secondary cracking |
| Carrier Gas Stream | Vapor Evacuation | Efficiently moves volatiles to the condensation system |
| Anaerobic Shield | Oxygen Displacement | Maintains zero-valent states in metallic nanoparticles |
Maximize Your Material Purity with KINTEK Precision
Don't let oxidation or secondary cracking compromise your research. KINTEK provides industry-leading high-temperature lab solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to your specific gas-flow requirements. Backed by our expert R&D and manufacturing, we help you achieve the precise anaerobic environments and flow control necessary for high-yield pyrolysis and catalyst synthesis.
Ready to upgrade your laboratory capabilities? Contact us today to discuss your custom furnace needs.
Visual Guide
References
- Hussien Elshareef, Yuguang Zhou. Investigation of Bio-Oil and Biochar Derived from Cotton Stalk Pyrolysis: Effect of Different Reaction Conditions. DOI: 10.3390/resources14050075
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What role does a laboratory tube furnace perform during the carbonization of LCNSs? Achieve 83.8% Efficiency
- What is an example of a material prepared using a tube furnace? Master Precise Material Synthesis
- How does a vertical tube furnace achieve precise temperature control? Unlock Superior Thermal Stability for Your Lab
- How do vertical tube furnaces comply with environmental standards? A Guide to Clean, Efficient Operation
- What are the key operational considerations when using a lab tube furnace? Master Temperature, Atmosphere & Safety



















