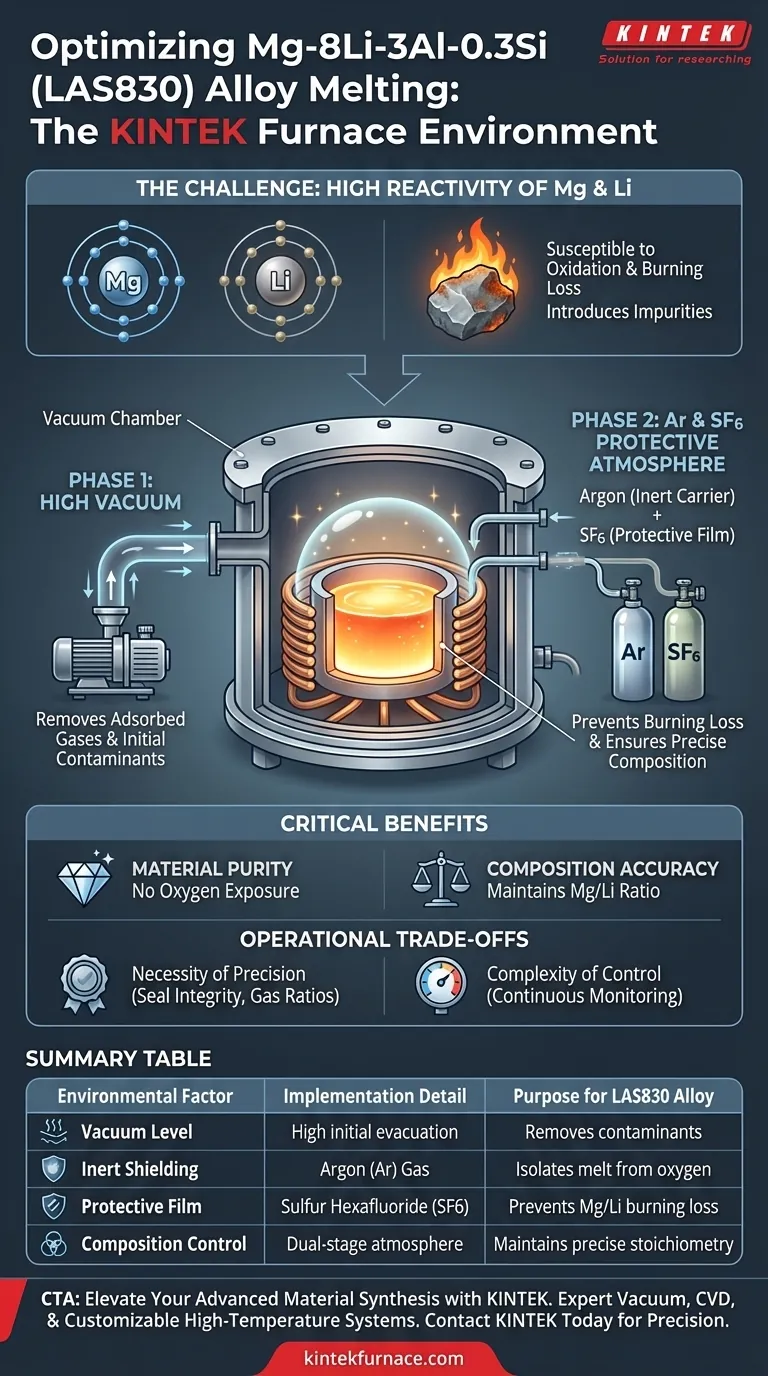
A vacuum induction resistance furnace provides a strictly controlled, dual-stage environment defined by a high vacuum and a specialized protective atmosphere. For the preparation of Mg-8Li-3Al-0.3Si (LAS830) alloys, this system specifically employs a mixture of Argon (Ar) and Sulfur Hexafluoride (SF6) to isolate the molten metal from atmospheric contaminants.
The extreme reactivity of Magnesium and Lithium makes them highly prone to oxidation and burning loss. This furnace setup ensures the precise chemical composition of the alloy by eliminating oxygen exposure and preventing the formation of impurities during the melting process.

The Critical Challenge: Reactivity of Mg and Li
High Chemical Activity
The primary difficulty in processing LAS830 lies in the nature of its core components.
Both Magnesium (Mg) and Lithium (Li) are highly chemically active metals.
Susceptibility to Oxidation
Under standard atmospheric conditions, these elements react rapidly with oxygen.
Without strict environmental controls, this leads to significant oxidation loss, altering the target ratio of the alloy elements.
Introduction of Impurities
Beyond the loss of material, reaction with air introduces unwanted oxides and impurities into the melt.
These impurities can compromise the structural integrity and mechanical properties of the final alloy.
How the Furnace Environment Protects the Alloy
strictly Controlled Vacuum
The first line of defense is the creation of a vacuum environment prior to and during specific stages of processing.
This effectively evacuates ambient air and removes adsorbed gases from the raw materials, establishing a baseline of purity.
The Ar and SF6 Protective Atmosphere
To actively protect the melt, the furnace introduces a specific mixture of Argon (Ar) and Sulfur Hexafluoride (SF6).
Argon acts as an inert carrier, while SF6 often facilitates the formation of a thin, protective film on the melt surface.
This gas combination prevents the active elements from reacting with any residual oxygen, ensuring the precise composition of the LAS830 alloy is maintained.
Understanding the Operational Trade-offs
Necessity of Precision
The effectiveness of this process relies entirely on the integrity of the seal and gas mixture ratios.
A failure in the vacuum seal or an imbalance in the Ar/SF6 mixture will result in immediate compositional drift due to the volatility of Lithium.
Complexity of Control
Unlike melting stable metals, processing LAS830 requires continuous monitoring of the atmosphere.
Operators must strictly control the vacuum levels and gas flow rates to balance protection against the risk of evaporating volatile elements under high vacuum.
Making the Right Choice for Your Goal
To ensure the successful preparation of LAS830 alloys, consider your specific priorities:
- If your primary focus is Compositional Accuracy: Prioritize the precise control of the Ar and SF6 gas mixture, as this prevents the burning loss of volatile Magnesium and Lithium.
- If your primary focus is Material Purity: Ensure the furnace is capable of achieving a high vacuum initially to fully outgas raw materials and remove adsorbed contaminants before melting begins.
By strictly controlling this dual environment, you secure both the purity and the precise stoichiometry required for high-performance LAS830 alloys.
Summary Table:
| Environmental Factor | Implementation Detail | Purpose for LAS830 Alloy |
|---|---|---|
| Vacuum Level | High initial evacuation | Removes adsorbed gases and prevents initial contamination |
| Inert Shielding | Argon (Ar) Gas | Acts as a stable carrier to isolate the melt from oxygen |
| Protective Film | Sulfur Hexafluoride (SF6) | Forms a surface barrier to prevent burning loss of Mg and Li |
| Composition Control | Dual-stage atmosphere | Maintains precise stoichiometry of highly reactive elements |
Elevate Your Advanced Material Synthesis with KINTEK
Processing reactive alloys like LAS830 requires uncompromising environmental control. At KINTEK, we provide industry-leading Vacuum, CVD, and customizable high-temperature furnace systems engineered to handle the volatility of Magnesium and Lithium.
Backed by expert R&D and manufacturing, our systems ensure your research or production achieves maximum material purity and precise chemical composition. Whether you need a standard solution or a unique, customizable setup, our team is ready to support your unique metallurgical needs.
Ready to optimize your melting process? Contact our experts today and let KINTEK deliver the precision your laboratory demands!
Visual Guide
References
- Changzhen Jia, Pengcheng Tian. Microstructure and Mechanical Properties of Mg-8Li-3Al-0.3Si Alloy Deformed Through a Combination of Back-Extrusion and Spinning Process. DOI: 10.3390/ma18020417
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Sintering and Brazing Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- Why is an induction melting furnace useful with ultrasonic atomization? Achieve Superior Metal Powder Quality
- What factors should be considered when selecting an induction melting furnace? A Guide to Maximizing ROI
- What role does a vacuum induction melting furnace play in the modification of W18Cr4V steel? Enhance Alloy Purity
- How does vacuum or protective atmosphere melting improve alloy composition uniformity? Achieve Precise Alloy Chemistry Control
- What role does a vacuum induction furnace with a water-cooled copper cold crucible play in melting Ti-33Mo-0.2C alloy?
- How do induction furnaces enhance productivity in foundries? Boost Melting Speed and Automation for Higher Output
- What are the key components of a vacuum casting furnace? Essential Parts for High-Purity Metal Casting
- What is the function of an industrial vacuum induction melting furnace in the directional solidification of blades?



















