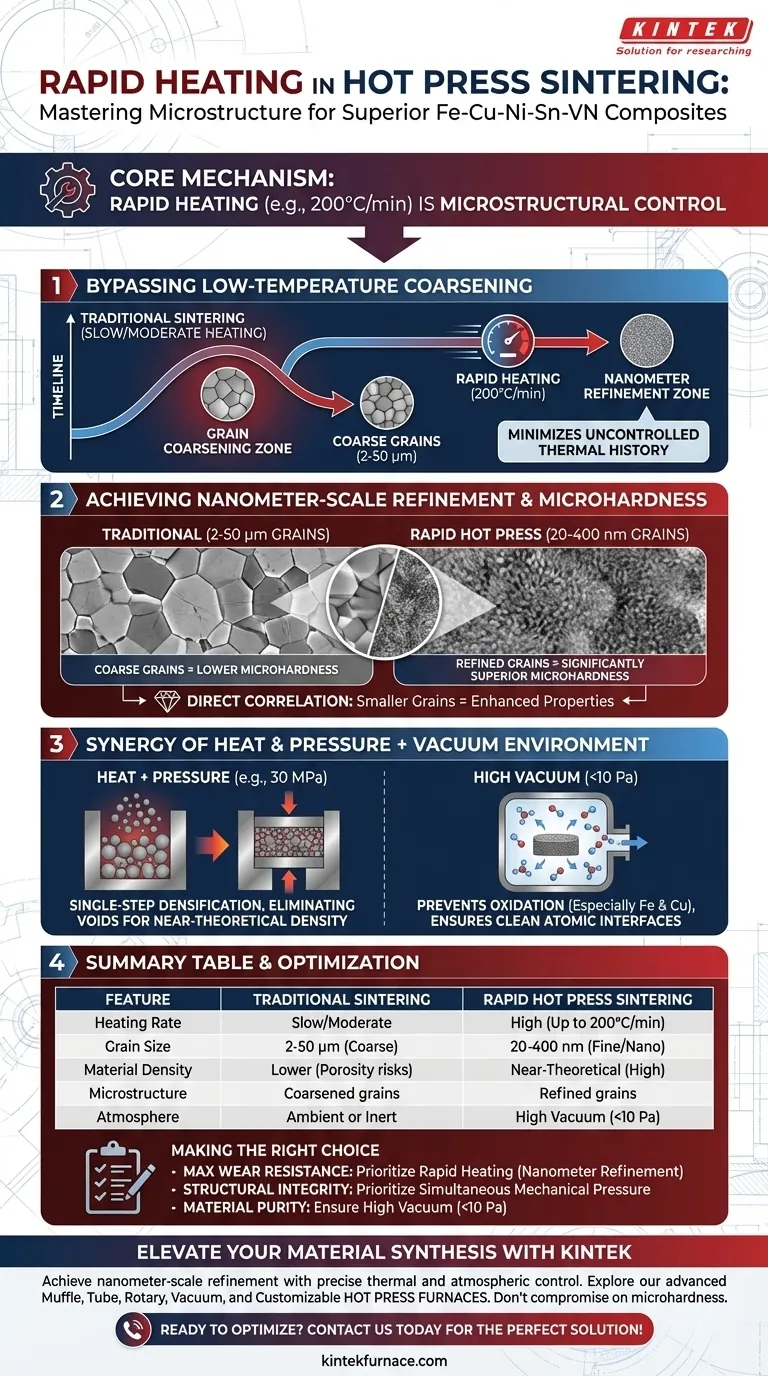
The primary significance of rapid heating in a hot press furnace is its ability to drastically minimize the material's uncontrolled thermal history. By escalating the temperature at rates such as 200°C/min, the process bypasses the low-temperature zones where grain coarsening typically occurs, directly enabling the refinement of grains from the micron level down to the nanometer level.
Core Takeaway Rapid heating is not merely a time-saver; it is a microstructural control mechanism. By combining high heating rates with simultaneous pressure, you suppress grain growth and maximize density, resulting in Fe-Cu-Ni-Sn-VN composites with significantly superior microhardness compared to traditional sintering methods.

Controlling Microstructure via Thermal History
Bypassing Low-Temperature Coarsening
In traditional sintering, prolonged exposure to lower temperatures allows grains to merge and grow larger. Rapid heating minimizes this exposure, quickly pushing the material to its optimal holding temperature. This speed effectively "locks in" a finer structure before the grains have time to coarsen.
Achieving Nanometer-Scale Refinement
The most critical outcome of this thermal control is grain refinement. While traditional methods often yield coarse grains between 2-50 microns, the rapid heating capability of a hot press furnace facilitates the formation of grains in the 20-400 nm range.
Direct Impact on Microhardness
There is a direct correlation between grain size and mechanical properties. The shift from micron-sized grains to nanometer-sized grains significantly enhances the microhardness of the Fe-Cu-Ni-Sn-VN composite.
The Synergy of Heat and Pressure
Single-Step Densification
Unlike cold pressing followed by sintering, a hot press furnace combines thermal energy and mechanical pressure (e.g., 30 MPa) simultaneously. This forces powder particles to rearrange and undergo plastic deformation while they are most malleable.
Eliminating Voids
The combination of heat and pressure effectively closes the gaps between particles. This leads to near-theoretical density, avoiding the porosity issues often found in pressureless sintering.
The Role of the Vacuum Environment
Preventing Oxidation
Fe-Cu-Ni-Sn-VN composites contain metals highly susceptible to oxidation, particularly iron and copper. The vacuum environment (often below 10 Pa) removes oxygen, preventing the formation of brittle oxide layers that would weaken the material.
Ensuring Clean Interfaces
By removing adsorbed gases from the powder surfaces, the vacuum ensures clean atomic interfaces. This promotes effective diffusion and solid solution formation between the different metallic elements, which is essential for high interfacial bonding strength.
Understanding the Trade-offs
Equipment Complexity vs. Material Quality
The primary trade-off is between process simplicity and material performance. Traditional "cold press and sinter" methods are simpler but result in coarser grains and lower wear resistance.
Precision Requirements
Achieving heating rates of 200°C/min requires advanced furnace capabilities and precise control systems. If the ramp rate is inconsistent, you risk introducing thermal gradients that could lead to nonuniform properties, though this risk is generally outweighed by the benefits of grain refinement.
Making the Right Choice for Your Goal
When optimizing the sintering process for Fe-Cu-Ni-Sn-VN composites, consider your specific performance requirements:
- If your primary focus is maximum wear resistance: Prioritize the rapid heating capability (200°C/min) to ensure nanometer-scale grain refinement and high microhardness.
- If your primary focus is structural integrity: Rely on the simultaneous application of mechanical pressure during the heating phase to eliminate voids and maximize density.
- If your primary focus is material purity: Ensure your furnace maintains a high vacuum (<10 Pa) to prevent oxidation of the iron and vanadium nitride components.
Rapid heating transforms the sintering process from a simple bonding step into a precise tool for nanostructural engineering.
Summary Table:
| Feature | Traditional Sintering | Rapid Hot Press Sintering |
|---|---|---|
| Heating Rate | Slow/Moderate | High (Up to 200°C/min) |
| Grain Size | 2-50 μm (Coarse) | 20-400 nm (Fine/Nano) |
| Material Density | Lower (Porosity risks) | Near-Theoretical (High) |
| Microstructure | Coarsened grains | Refined grains |
| Atmosphere | Ambient or Inert | High Vacuum (<10 Pa) |
Elevate Your Material Synthesis with KINTEK
Achieving nanometer-scale refinement in Fe-Cu-Ni-Sn-VN composites requires more than just heat; it requires precise control over thermal history and atmosphere. Backed by expert R&D and manufacturing, KINTEK offers advanced Muffle, Tube, Rotary, Vacuum, and CVD systems, as well as high-performance Hot Press furnaces customizable for your unique research and production needs.
Don't let grain coarsening compromise your material's microhardness. Our specialized lab high-temp furnaces provide the rapid heating rates and vacuum integrity necessary to push the boundaries of materials science.
Ready to optimize your sintering process? Contact us today to find the perfect solution!
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What functions does a graphite mold perform? Unlock Superior Densification in Vacuum Hot Pressing
- Why use Vacuum Hot Press (VHP) for ZnS Ceramics? Achieve Superior IR Transparency and Mechanical Strength
- What are the advantages of a vacuum hot pressing sintering furnace for rare earth copper composites? Density & Purity
- What is activated hot sintering and its advantages? Achieve Superior Material Densification Efficiently
- What are the primary applications of vacuum press technology? Achieve Superior Material Bonding and Shaping
- What types of advanced materials can be prepared using a vacuum press? Unlock High-Performance Fabrication
- Why are high-purity graphite sleeves used in Multi-anvil Presses? Unlock 2300°C Precision and Reducing Environments
- How does precise temperature control in a Vacuum Hot Press Furnace influence the microstructure of Al-Ti system materials? Achieve Superior Microstructural Integrity



















