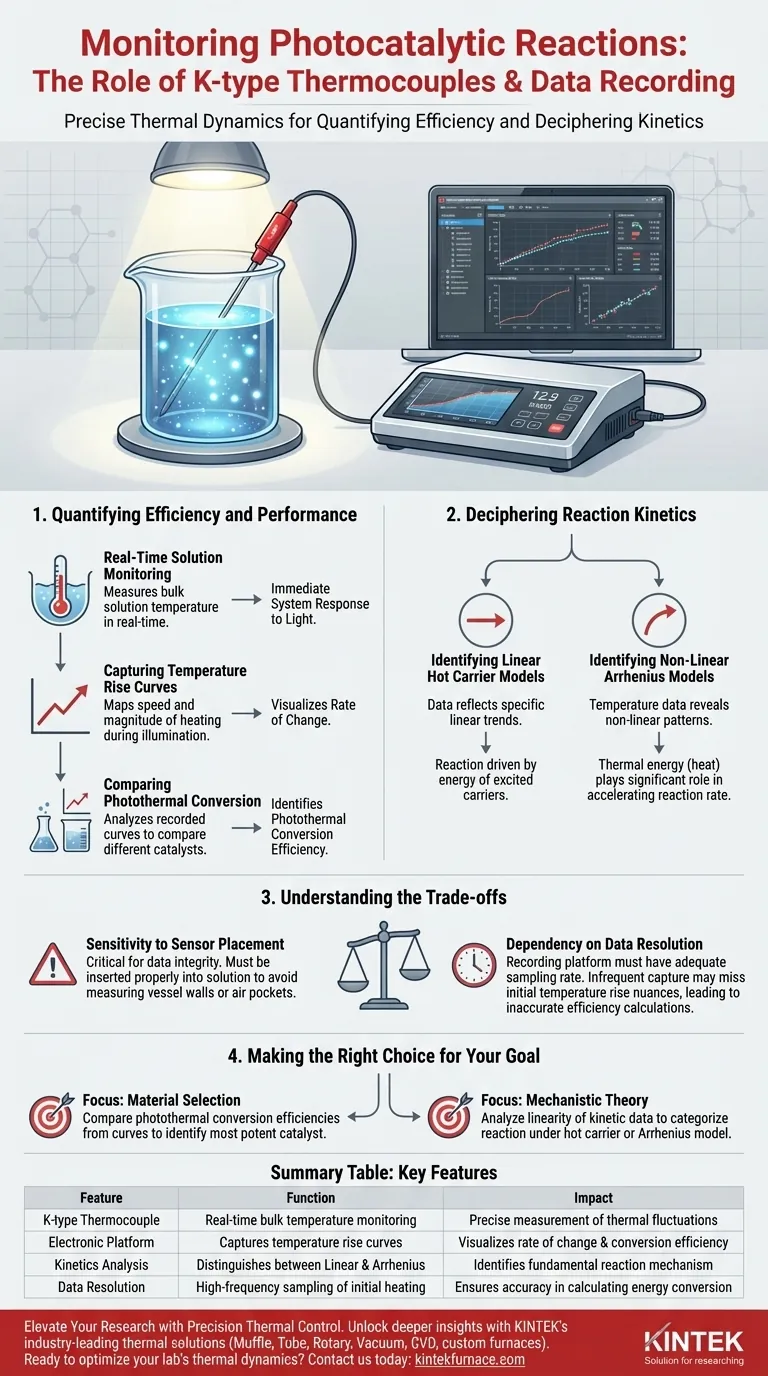
The primary role of K-type thermocouples and electronic data recording platforms is to provide precise, real-time quantification of thermal dynamics within photocatalytic reactions. By inserting the thermocouple directly into the reaction vessel, researchers can monitor solution temperature fluctuations, while the electronic platform captures detailed temperature rise curves during illumination to evaluate system efficiency.
Precise thermal data is the bridge between observing a reaction and understanding its mechanism. It allows researchers to move beyond simple observation and quantitatively determine whether a reaction is driven by linear hot carrier dynamics or non-linear photothermal enhancement.

Quantifying Efficiency and Performance
Real-Time Solution Monitoring
K-type thermocouples are specifically utilized to measure the bulk temperature of the solution inside the reaction vessel.
Because these measurements happen in real-time, they provide an immediate readout of how the system responds to light exposure.
Capturing Temperature Rise Curves
The electronic data recording platform is essential for visualizing the rate of change over time.
It records the "temperature rise curve," which maps the speed and magnitude of heating during the illumination phase.
Comparing Photothermal Conversion
By analyzing these recorded curves, researchers can compare different catalysts side-by-side.
This data reveals the photothermal conversion efficiency of each specific catalyst, identifying which materials are most effective at converting light energy into thermal energy.
Deciphering Reaction Kinetics
Identifying Linear Hot Carrier Models
The data collected is critical for modeling reaction kinetics.
If the recorded data reflects specific linear trends, it suggests the reaction follows a linear hot carrier model, where the reaction is driven primarily by the energy of excited carriers.
Identifying Non-Linear Arrhenius Models
Conversely, the temperature data may reveal non-linear patterns.
These patterns indicate that the reaction follows a non-linear Arrhenius photothermal enhancement model, suggesting that thermal energy (heat) plays a significant role in accelerating the reaction rate.
Understanding the Trade-offs
Sensitivity to Sensor Placement
While K-type thermocouples are robust, their placement is critical for data integrity.
The sensor must be inserted properly into the solution; otherwise, it may record the temperature of the vessel walls or air pockets rather than the reaction medium itself.
Dependency on Data Resolution
The electronic recording platform is only as useful as its sampling rate.
If the platform cannot capture data points frequently enough, it may miss the nuances of the initial temperature rise, leading to inaccurate calculations of conversion efficiency.
Making the Right Choice for Your Goal
To maximize the value of your experimental setup, align your analysis with your specific research objectives:
- If your primary focus is material selection: Compare the photothermal conversion efficiencies derived from temperature rise curves to identify the most potent catalyst.
- If your primary focus is mechanistic theory: Analyze the linearity of your kinetic data to definitively categorize the reaction under a hot carrier or Arrhenius model.
Accurate thermal data is the key to distinguishing between simple heating and complex catalytic behavior.
Summary Table:
| Feature | Function in Photocatalytic Research | Impact on Data Quality |
|---|---|---|
| K-type Thermocouple | Real-time bulk solution temperature monitoring | Precise measurement of thermal fluctuations |
| Electronic Platform | Captures temperature rise curves during illumination | Visualizes rate of change and conversion efficiency |
| Kinetics Analysis | Distinguishes between Linear Hot Carrier vs. Arrhenius models | Identifies the fundamental reaction mechanism |
| Data Resolution | High-frequency sampling of initial heating phases | Ensures accuracy in calculating energy conversion |
Elevate Your Research with Precision Thermal Control
Unlock deeper insights into your photocatalytic reactions with KINTEK’s industry-leading thermal solutions. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, along with customizable lab high-temp furnaces tailored to your unique experimental needs.
Whether you are analyzing photothermal conversion efficiency or deciphering complex reaction kinetics, our precision-engineered equipment ensures the accuracy and reliability your data demands.
Ready to optimize your lab's thermal dynamics? Contact us today to discuss your custom furnace requirements with our specialists.
Visual Guide
References
- Dreenan Shea, Mita Dasog. Decoding Plasmonic Enhancement Pathways in Group 4 Metal Nitride‐TiO<sub>2</sub> Composites: Rhodamine B Dye Degradation Case Study. DOI: 10.1002/nano.70059
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- What are resistance heating elements made of and where are they used? Discover Materials for Efficient Heat Generation
- What is the maximum temperature for a quartz heating element? Up to 2400°C in Open Air
- What is the operating temperature range for Nichrome heating elements? Maximize Lifespan and Performance
- How do SIC heating elements perform in harsh environments? Unlock Durability and Efficiency in Extreme Conditions
- Which heating element has better oxidation resistance? Discover MoSi2's Superior Protection
- What are the advantages of MoSi2 heating elements in terms of efficiency? Maximize High-Temp Process Speed & Energy Savings
- What maintenance considerations are important for furnace heating elements? Ensure Longevity and Reliability
- Do ceramic heaters come with timers? Find the Best Timer and Thermostat Options



















