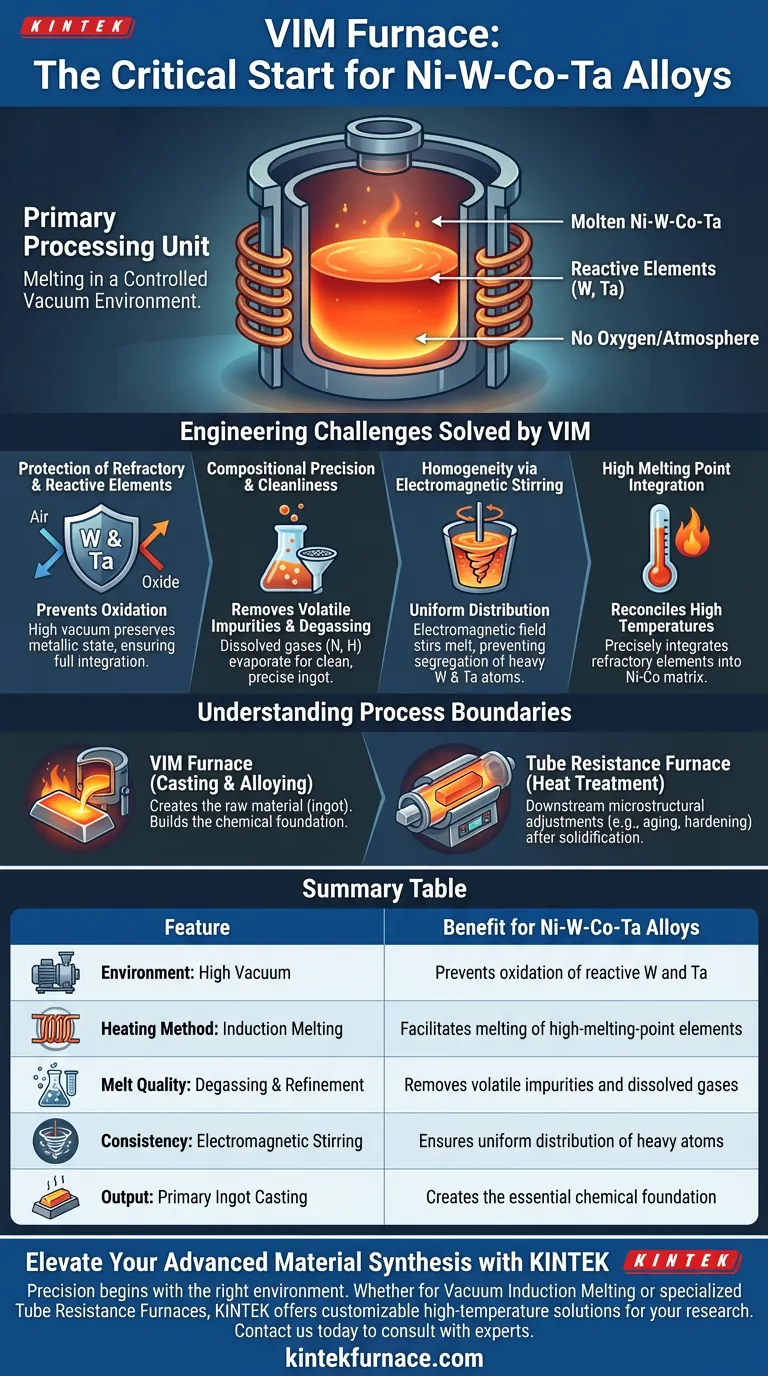
The Vacuum Induction Melting (VIM) furnace serves as the critical primary processing unit for the preparation of Ni-W-Co-Ta alloys. Its specific function is to perform the initial melting and alloying within a controlled vacuum environment, which is essential to prevent the oxidation of reactive, high-melting-point elements such as tungsten (W) and tantalum (Ta). By isolating the melt from atmospheric oxygen, the VIM furnace ensures the production of clean, compositionally accurate initial ingots.
The central value of the VIM furnace lies in its ability to reconcile high melting temperatures with chemical purity. It allows for the precise integration of refractory elements like tungsten and tantalum into the nickel-cobalt matrix without the risk of oxide formation or contamination.
The Engineering Challenges Solved by VIM
The preparation of complex alloys like Ni-W-Co-Ta presents specific metallurgical hurdles that standard melting techniques cannot address. The VIM furnace overcomes these through vacuum protection and induction mechanics.
Protection of Refractory and Reactive Elements
Tungsten and tantalum are characterized by both high melting points and high chemical activity.
In the presence of air, these elements would rapidly oxidize at melting temperatures. The VIM furnace eliminates this risk by operating under a high vacuum. This environment preserves the metallic state of tungsten and tantalum, ensuring they are fully integrated into the alloy rather than lost as slag or oxide inclusions.
Compositional Precision and Cleanliness
Achieving the exact chemical ratio in Ni-W-Co-Ta is vital for the alloy's final properties.
The VIM process allows for the removal of volatile impurities through degassing. As the metal melts in a vacuum, dissolved gases (such as nitrogen and hydrogen) and high-vapor-pressure impurities evaporate from the melt. This results in a "clean" initial ingot with tightly controlled composition.
Homogeneity via Electromagnetic Stirring
While the primary reference focuses on oxidation prevention, the mechanics of VIM offer a secondary benefit critical for heavy elements.
Induction heating generates an electromagnetic field that naturally stirs the molten metal. For alloys containing high-mass solute elements like tungsten and tantalum, this stirring effect prevents segregation. It ensures these heavy atoms are uniformly distributed throughout the lighter nickel-cobalt matrix, establishing a high-quality foundation for subsequent processing.
Understanding the Process Boundaries
It is important to distinguish the role of the VIM furnace from other thermal processing equipment used later in the alloy's lifecycle.
Melting vs. Heat Treatment
The VIM furnace is strictly for the casting and alloying phase. It creates the raw material (the ingot).
It is not used for downstream microstructural adjustments. For example, after the alloy has been cold rolled, a laboratory high-temperature tube resistance furnace is typically used. That equipment handles the lower-temperature (e.g., 700°C) aging processes required to precipitate strengthening phases (like Ni4W). The VIM furnace builds the chemical foundation; the resistance furnace optimizes the physical microstructure.
Making the Right Choice for Your Goal
When planning the production line for Ni-W-Co-Ta alloys, distinct equipment serves distinct metallurgical objectives:
- If your primary focus is Chemical Integrity: Rely on the VIM furnace to melt and alloy reactive elements (W, Ta) without oxidation or atmospheric contamination.
- If your primary focus is Microstructural Hardening: Utilize a tube resistance furnace to control precipitation reactions and grain size after the material has been solidified and rolled.
Summary: The VIM furnace is the non-negotiable starting point for Ni-W-Co-Ta production, ensuring that high-value refractory elements are successfully alloyed into a pure, homogeneous ingot.
Summary Table:
| Feature | VIM Furnace Role | Benefit for Ni-W-Co-Ta Alloys |
|---|---|---|
| Environment | High Vacuum | Prevents oxidation of reactive W and Ta |
| Heating Method | Induction Melting | Facilitates melting of high-melting-point refractory elements |
| Melt Quality | Degassing & Refinement | Removes volatile impurities and dissolved gases |
| Consistency | Electromagnetic Stirring | Ensures uniform distribution of heavy atoms in the matrix |
| Output | Primary Ingot Casting | Creates the essential chemical foundation for later processing |
Elevate Your Advanced Material Synthesis with KINTEK
Precision in Ni-W-Co-Ta alloy production begins with the right thermal environment. Whether you need to achieve chemical purity through Vacuum Induction Melting or optimize microstructures with our specialized Tube Resistance Furnaces, KINTEK provides the engineering excellence your laboratory requires.
Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. All our lab high-temp furnaces are fully customizable to meet your unique metallurgical challenges and research specifications.
Ready to refine your alloying process? Contact KINTEK today to consult with our experts and find the perfect high-temperature solution for your needs.
Visual Guide
References
- Yong Li, Chunxu Wang. Effect of Aging Time on Microstructure and Properties of Cold-Rolled Ni-W-Co-Ta Medium–Heavy Alloy. DOI: 10.3390/coatings14020230
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
People Also Ask
- What are the main benefits of using an induction furnace for gold melting compared to traditional furnaces? Discover Faster, Purer Melts
- Why is a Vacuum Induction Melting (VIM) furnace essential? Unlock Purity for Aerospace and Semiconductors
- What crucible materials are used in IGBT induction melting furnaces for different metals? Select the Right Crucible for a Clean, High-Quality Melt
- What advantages does vacuum induction melting offer? Achieve Unmatched Metal Purity and Performance
- What are the key components of an induction-heated vacuum furnace? Uncover the Systems for Pure Melting
- How does an integrated system of in-situ neutron diffraction, high-frequency induction heating, and deformation devices address technical challenges in metallurgical research? Uncover Real-Time Microstructural Evolution
- How do channel induction furnaces improve the working environment? Achieve a Safer, Cleaner, and Quieter Workplace
- How does a vacuum induction melting furnace (VIM furnace) work? Achieve Ultra-Pure Metals with Precision Melting



















