A laboratory vacuum drying oven plays a dual role in fluoride-ion battery material preparation: it ensures the absolute chemical purity of sensitive precursors through deep dehydration and facilitates the structural integrity of composite coatings by removing solvents. Specifically, it eliminates trace moisture from Bismuth Fluoride (BiF3) and Tin (Sn) components to prevent oxidation, while also aiding the encapsulation process in polymer-coated composites.
By creating a heated, low-pressure environment, vacuum drying prevents the formation of oxygen impurities and enables dense protective structures, both of which are fundamental to the stability and efficiency of the final battery cell.
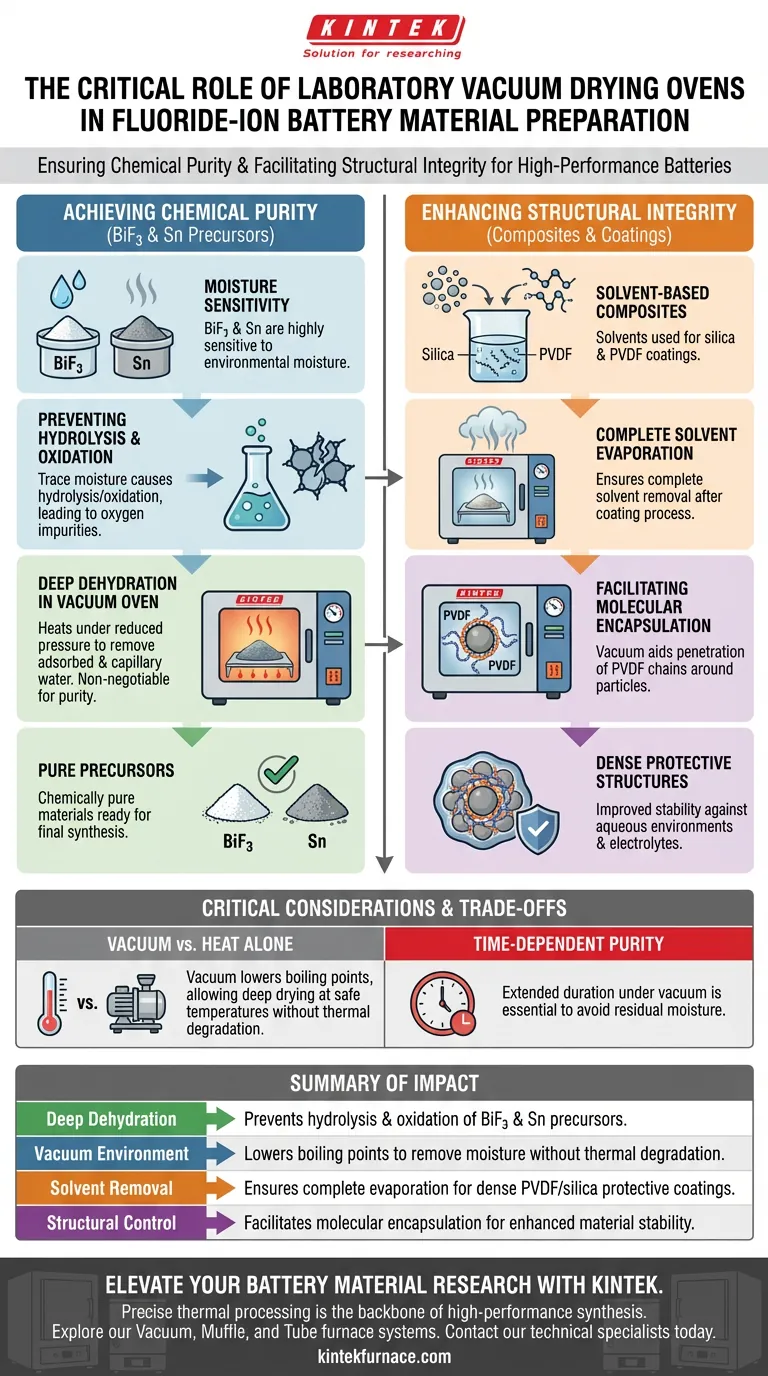
Achieving Chemical Purity Through Deep Dehydration
Eliminating Moisture Sensitivity
Fluoride-ion battery precursors, particularly BiF3 cathode materials and Sn anode powders, are highly sensitive to environmental moisture. Even trace amounts of water vapor can initiate detrimental chemical changes.
Preventing Hydrolysis and Oxidation
If moisture is present during synthesis, it can lead to hydrolysis or oxidation of the raw materials. This results in unwanted oxygen impurities that degrade the electrochemical performance of the battery.
Removing Adsorbed Water
The vacuum drying oven heats materials under reduced pressure to thoroughly remove both adsorbed and capillary water. This step is non-negotiable for ensuring the precursors remain chemically pure prior to the final synthesis.
Enhancing Structural Integrity in Composites
Complete Solvent Evaporation
In the preparation of complex composites—such as those involving silica coatings or polyvinylidene fluoride (PVDF) layers—solvents are used to dissolve polymers. The vacuum oven ensures these solvents are completely evaporated after the coating process.
Facilitating Molecular Encapsulation
Beyond simple drying, the vacuum environment aids the physical formation of the material. It facilitates the penetration of PVDF molecular chains around the outer layer of particles.
Creating Protective Layers
This process helps create a dense, dual-protective structure. By ensuring tight encapsulation, the material gains significantly improved stability, particularly against aqueous environments or electrolyte interactions.
Critical Considerations and Trade-offs
The Necessity of Vacuum vs. Heat Alone
Using heat without a vacuum is often insufficient for these materials. A vacuum lowers the boiling point of water and solvents, allowing for deep drying at temperatures that won't thermally degrade the sensitive polymer components or alter the crystal structure of the fluoride salts.
Time-Dependent Purity
The process is not instantaneous. The primary reference notes that materials must be heated under vacuum for extended periods. Rushing this step increases the risk of residual moisture, which will inevitably compromise the battery's cycle life and capacity.
Making the Right Choice for Your Goal
To maximize the effectiveness of your material preparation, align your drying protocol with your specific synthesis stage:
- If your primary focus is Precursor Purity (BiF3/Sn): Prioritize high vacuum levels and extended duration to eliminate all capillary water and prevent oxygen impurities.
- If your primary focus is Composite Stability (PVDF/Coatings): Focus on the solvent evaporation phase to ensure dense molecular chain penetration and robust encapsulation.
The vacuum drying oven is not merely a drying tool; it is a synthesis instrument that defines the chemical purity and structural architecture of high-performance battery materials.
Summary Table:
| Feature | Impact on Fluoride-Ion Battery Materials |
|---|---|
| Deep Dehydration | Prevents hydrolysis and oxidation of BiF3 and Sn precursors. |
| Vacuum Environment | Lowers boiling points to remove moisture without thermal degradation. |
| Solvent Removal | Ensures complete evaporation for dense PVDF/silica protective coatings. |
| Structural Control | Facilitates molecular encapsulation for enhanced material stability. |
Elevate Your Battery Material Research with KINTEK
Precise thermal processing is the backbone of high-performance battery synthesis. KINTEK provides industry-leading Vacuum, Muffle, and Tube furnace systems specifically designed to meet the rigorous purity standards of fluoride-ion technology.
Backed by expert R&D and manufacturing, our lab solutions are fully customizable—ensuring your sensitive precursors and composite coatings achieve maximum stability and electrochemical efficiency.
Ready to optimize your lab's drying and synthesis protocols? Contact our technical specialists today to discuss your unique needs.
Visual Guide
References
- Hong Chen, Oliver Clemens. Revealing an Intercalation Nature of High‐Capacity Conversion Cathode Materials for Fluoride‐Ion Batteries by Operando Studies. DOI: 10.1002/smtd.202500374
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering and Brazing Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the primary function of a high vacuum drying oven in B4C/Al powder pretreatment? Protect Purity & Prevent Pores
- What are the advantages of the re-coating process? Boost Adsorbent Capacity Beyond Original Performance
- Why is the calcination process essential for Fe3O4/CeO2 and NiO/Ni@C? Control Phase Identity and Conductivity
- What is the purpose of using a vacuum dryer for PU and AlN composite sheets? Enhance Thermal & Structural Integrity
- How does the speed-controlled motor in a high-pressure autoclave influence the yield of glucose from starch?
- How do vertical reaction furnaces simulate blast furnace reduction? Recover Iron from Steel Waste Effectively
- What role does a laboratory oven play in W-doped TiO2? Ensure Precursor Stability for High-Purity Nanopowders
- What are the advantages of Spark Plasma Sintering (SPS) for TiC alloys? Preserve Precision and Microstructure



















