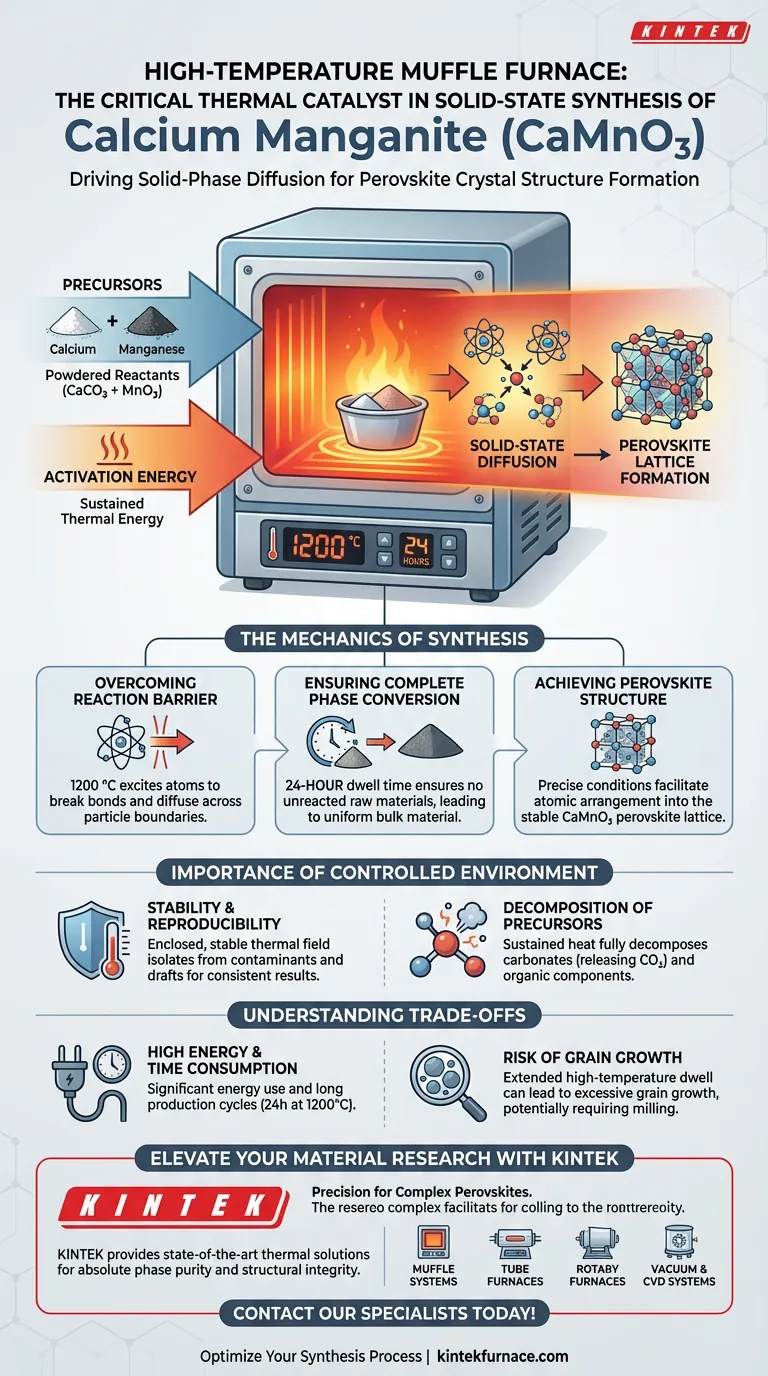
A high-temperature muffle furnace acts as the critical thermal catalyst in the solid-state synthesis of Calcium Manganite (CaMnO3). It provides a stable, isolated environment capable of maintaining extreme temperatures—typically 1200 °C—for extended periods, often up to 24 hours. This sustained thermal energy is required to drive solid-phase diffusion between precursors like calcium carbonate and manganese oxide, ensuring they fully react to form the desired perovskite crystal structure.
Core Insight: The muffle furnace does not merely heat the materials; it provides the activation energy required for solid-state diffusion. Without this precise, high-temperature environment (1200 °C) maintained over a long duration, the raw precursors would remain a physical mixture rather than chemically bonding into a single-phase, high-purity perovskite lattice.
The Mechanics of Solid-State Synthesis
Overcoming the Reaction Barrier
In solid-state synthesis, the reactants are powders, meaning atoms must physically move (diffuse) across particle boundaries to react. This process is naturally slow and energy-intensive.
The muffle furnace provides the necessary 1200 °C environment to overcome this kinetic barrier. This high thermal energy excites the atoms within the calcium carbonate and manganese oxide, allowing them to break their original bonds and diffuse into one another to form the new CaMnO3 compound.
Ensuring Complete Phase Conversion
The transformation from raw precursors to a finished ceramic is rarely instantaneous. The furnace facilitates a continuous heating process, often lasting 24 hours.
This extended duration is critical for "complete conversion." It ensures that no unreacted raw materials remain and that the reaction propagates through the entire bulk of the powder, resulting in a uniform material.
Achieving Specific Crystal Structures
Calcium Manganite is a perovskite, a family of materials defined by a specific, complex crystal arrangement.
The muffle furnace ensures the material reaches the thermodynamic conditions necessary for the atoms to arrange themselves into this specific perovskite crystal structure. The stability of the furnace prevents temperature fluctuations that could lead to impurities or structural defects.
The Importance of a Controlled Environment
Stability and Reproducibility
A key feature of the muffle furnace is its ability to provide a stable thermal field.
Unlike open flames or uneven heating methods, a muffle furnace encloses the sample, isolating it from external contaminants and drafts. This isolation ensures that every batch receives the exact same thermal treatment, which is vital for scientific reproducibility and high phase purity.
Decomposition of Precursors
Before the final crystal structure forms, the raw materials often undergo decomposition. For example, carbonate precursors must release carbon dioxide.
The furnace provides the sustained heat required to fully decompose organic components or carbonates. This step effectively "cleans" the material, leaving behind only the metal oxides required for the final ceramic structure.
Understanding the Trade-offs
High Energy and Time Consumption
While effective, this method is resource-intensive. The requirement for 1200 °C temperatures over 24 hours demands significant energy consumption and prolongs the production cycle compared to other synthesis methods (like sol-gel or hydrothermal).
Risk of Grain Growth
Extended dwell times at high temperatures can lead to excessive grain growth. While the furnace ensures phase purity, the long heating cycle can sometimes result in larger particle sizes, which may require subsequent milling if a fine powder is the ultimate goal.
Making the Right Choice for Your Goal
To maximize the quality of your Calcium Manganite synthesis, tailor your furnace usage to your specific objectives:
- If your primary focus is Phase Purity: Ensure the furnace is programmed for the full 24-hour dwell time to guarantee the complete reaction of all precursors.
- If your primary focus is Crystallinity: Verify that the furnace can maintain a stable 1200 °C without fluctuation, as this specific temperature is the driver for forming the correct perovskite lattice.
- If your primary focus is Contamination Control: Utilize the enclosed nature of the muffle furnace to protect the sample from external impurities during the long sintering cycle.
Ultimately, the muffle furnace serves as the engine of solid-state synthesis, converting raw chemical potential into a structured, functional ceramic through precise thermal force.
Summary Table:
| Feature | Role in CaMnO3 Synthesis | Key Parameter |
|---|---|---|
| Operating Temperature | Provides activation energy for atomic diffusion | 1200 °C |
| Dwell Time | Ensures complete phase conversion & impurity removal | 24 Hours |
| Environment | Isolated chamber prevents contamination & fluctuations | Controlled/Stable |
| Structural Goal | Facilitates formation of specific perovskite lattice | High Phase Purity |
| Process Impact | Decomposes precursors (e.g., carbonates) | CO2 Release |
Elevate Your Material Research with KINTEK
Precision is non-negotiable when synthesizing complex perovskites like Calcium Manganite. KINTEK provides state-of-the-art thermal solutions engineered for researchers and manufacturers who demand absolute phase purity and structural integrity.
Backed by expert R&D and world-class manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you require the high-temperature stability of a standard muffle furnace for solid-state synthesis or a fully customizable high-temp system for unique laboratory needs, KINTEK delivers the reliability your work deserves.
Ready to optimize your synthesis process? Contact our specialists today to find the perfect furnace solution!
Visual Guide
References
- Mathias Pein, Christian Sattler. Thermochemical Oxygen Pumping with Perovskite Reticulated Porous Ceramics for Enhanced Reduction of Ceria in Thermochemical Fuel Production. DOI: 10.1002/aenm.202304454
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What are the typical uses of muffle furnaces in laboratory settings? Unlock Precise Heat Treatment for Your Lab
- Why is a high-precision multi-functional muffle furnace required in CGFS? Achieve Exact Decarbonization Metrics
- What types of analyses can be conducted using a muffle furnace? Essential High-Temperature Tests for Accurate Results
- Why is a muffle furnace with precise temperature control required for space holder removal? Ensure Structural Integrity
- What is the function of a muffle furnace in NiFe2O4/biochar prep? Optimize Your Composite Synthesis
- What atmosphere control options are available in advanced muffle furnaces? Master Materials Processing with Precision
- What role does a muffle furnace play in g-C3N4 synthesis? Mastering Thermal Polycondensation for Semiconductors
- How is a muffle furnace utilized in the two-stage calcination for C3N4 nanosheets? Precision Thermal Synthesis Guide



















