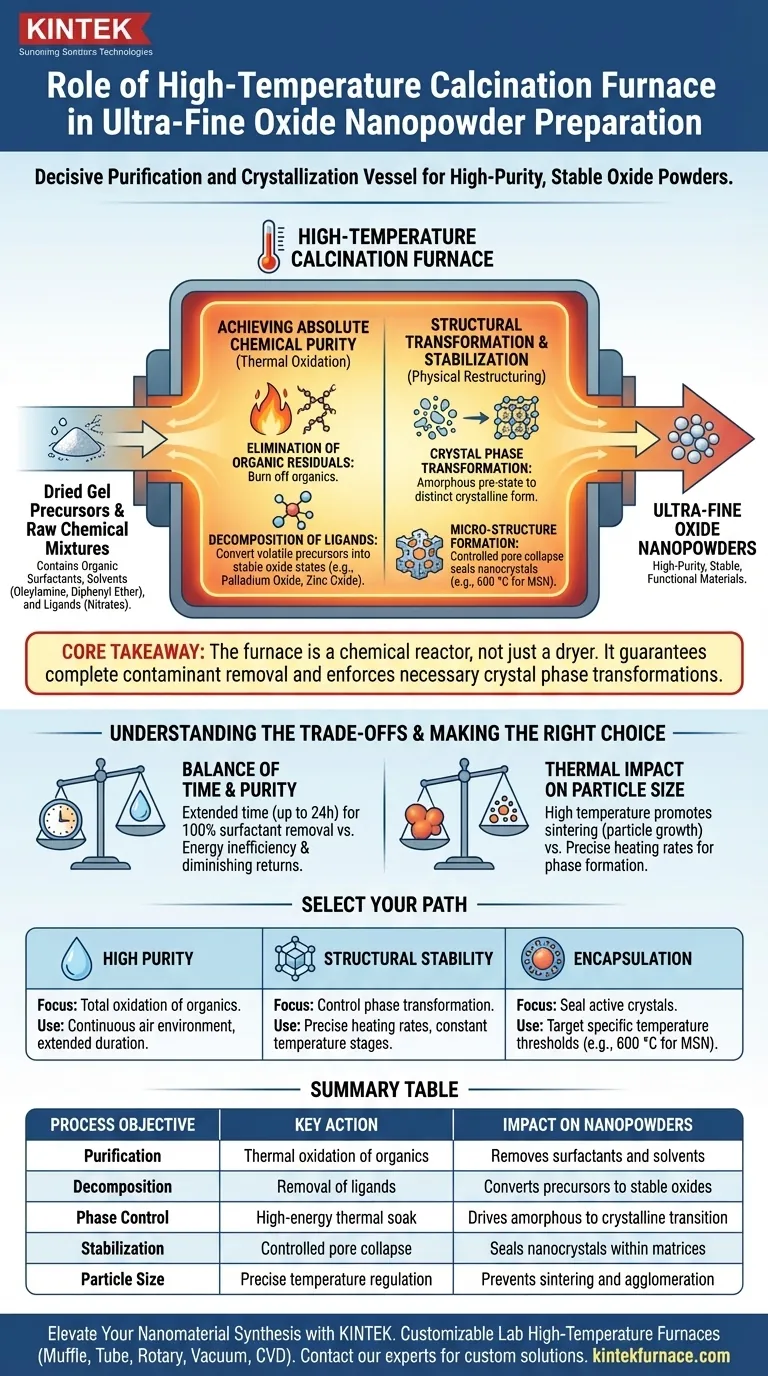
The high-temperature calcination furnace serves as the decisive purification and crystallization vessel in the synthesis of ultra-fine oxide nanopowders. By maintaining a continuous, high-temperature air environment—often for durations spanning 24 hours—the furnace drives the thermal oxidation of dried gel precursors to transform raw chemical mixtures into stable, functional materials.
Core Takeaway The calcination furnace does more than simply dry the material; it acts as a chemical reactor. Its primary function is to guarantee the complete removal of organic contaminants and enforce the necessary crystal phase transformations required for high-purity, stable oxide powders.

Achieving Absolute Chemical Purity
The initial role of the furnace is to strip away unwanted chemical artifacts left over from the synthesis process.
Elimination of Organic Residuals
During the precursor stage, materials often contain organic surfactants and solvents, such as oleylamine and diphenyl ether.
The high-temperature environment facilitates the thermal oxidation reaction required to burn off these organics completely.
Decomposition of Ligands
Beyond solvents, the furnace creates a controlled oxidation environment to decompose metal precursors adsorbed on the material support.
Specific ligands, such as nitrates or acetylacetonates, are removed during this stage. This conversion process is essential for turning volatile metal components into stable oxide states, such as palladium oxide or zinc oxide.
Structural Transformation and Stabilization
Once impurities are removed, the furnace energy drives the physical restructuring of the material.
Crystal Phase Transformation
The heat provided ensures the material undergoes a complete crystal phase transformation.
This shifts the material from a potentially amorphous or unstable pre-state into its final, distinct crystalline form. This step is critical for defining the physical properties of the ultra-fine powder.
Micro-Structure Formation
In specific applications, such as with Mesoporous Silica Nanoparticles (MSN), precise temperature control (e.g., at 600 °C) triggers in-situ crystallization within mesoporous channels.
At this specific thermal point, surface pores can undergo a partial collapse. This effectively seals generated nanocrystals inside the matrix, creating a highly stable protective layer.
Understanding the Trade-offs
While calcination is vital, it introduces critical variables that must be managed to avoid degrading the final product.
The Balance of Time and Purity
Extended calcination times (up to 24 hours) are often necessary to ensure 100% removal of surfactants.
However, excessive duration can lead to energy inefficiency without yielding significant additional purity, creating a point of diminishing returns.
Thermal Impact on Particle Size
The goal is to produce "ultra-fine" nanopowders, but high temperatures naturally promote sintering (particle growth).
If the temperature is too high or uncontrolled, the particles may fuse, ruining the "nano" characteristic. Precise heating rates are required to balance phase formation against unwanted particle agglomeration.
Making the Right Choice for Your Goal
The parameters of your calcination process should be dictated by the specific requirements of your final oxide powder.
- If your primary focus is High Purity: Prioritize a continuous air environment and extended duration to ensure the total oxidation of persistent organics like oleylamine.
- If your primary focus is Structural Stability: Utilize precise heating rates and constant temperature stages to control the phase transformation and lock in the micro-structure of active sites.
- If your primary focus is Encapsulation: Target specific temperature thresholds (e.g., 600 °C for MSN) to trigger pore collapse and seal active crystals within the support matrix.
Success in this stage depends on rigorously controlling the thermal environment to achieve purity without compromising the ultra-fine structure of the material.
Summary Table:
| Process Objective | Key Action in Furnace | Impact on Nanopowders |
|---|---|---|
| Purification | Thermal oxidation of organics | Removes surfactants (oleylamine) and solvents |
| Decomposition | Removal of ligands (nitrates) | Converts metal precursors into stable oxide states |
| Phase Control | High-energy thermal soak | Drives transition from amorphous to crystalline form |
| Stabilization | Controlled pore collapse | Seals nanocrystals within matrices (e.g., MSN) |
| Particle Size | Precise temperature regulation | Prevents sintering and unwanted agglomeration |
Elevate Your Nanomaterial Synthesis with KINTEK
Precise thermal control is the difference between a fused mass and a high-purity nanopowder. KINTEK provides the industry-leading R&D and manufacturing expertise required to master these delicate transformations. Whether you need a Muffle, Tube, Rotary, Vacuum, or CVD system, our lab high-temperature furnaces are fully customizable to meet your specific calcination profiles.
Ready to achieve absolute purity and stable crystal phases?
Contact our experts today to find your custom furnace solution
Visual Guide
References
- Lee Shelly, Shmuel Hayun. Unveiling the factors determining water adsorption on CeO <sub>2</sub> , ThO <sub>2</sub> , UO <sub>2</sub> and their solid solutions. DOI: 10.1007/s12598-025-03393-w
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What are the advantages of using an Infrared Rapid Heating Furnace? Capture Transient Atomic Migrations in Steel
- What is the role of Muffle or Tube furnaces in carbon nitride preparation? Optimize Your Thermal Polymerization
- How does a muffle furnace ensure precise temperature control? Discover the Key Components for Accurate Heating
- What types of processes can modern muffle furnaces support? Discover Versatile High-Temperature Solutions
- Why is an industrial muffle furnace required to process sugar beet samples at 550 °C for crude ash determination?
- How does an industrial muffle furnace facilitate the chemical activation of clay? Unlock High-Performance Zeolites
- How are muffle furnaces utilized in the ceramics industry? Essential for Precision Firing and Sintering
- What are the main components of a muffle furnace? Key Parts for Precision High-Temp Control



















