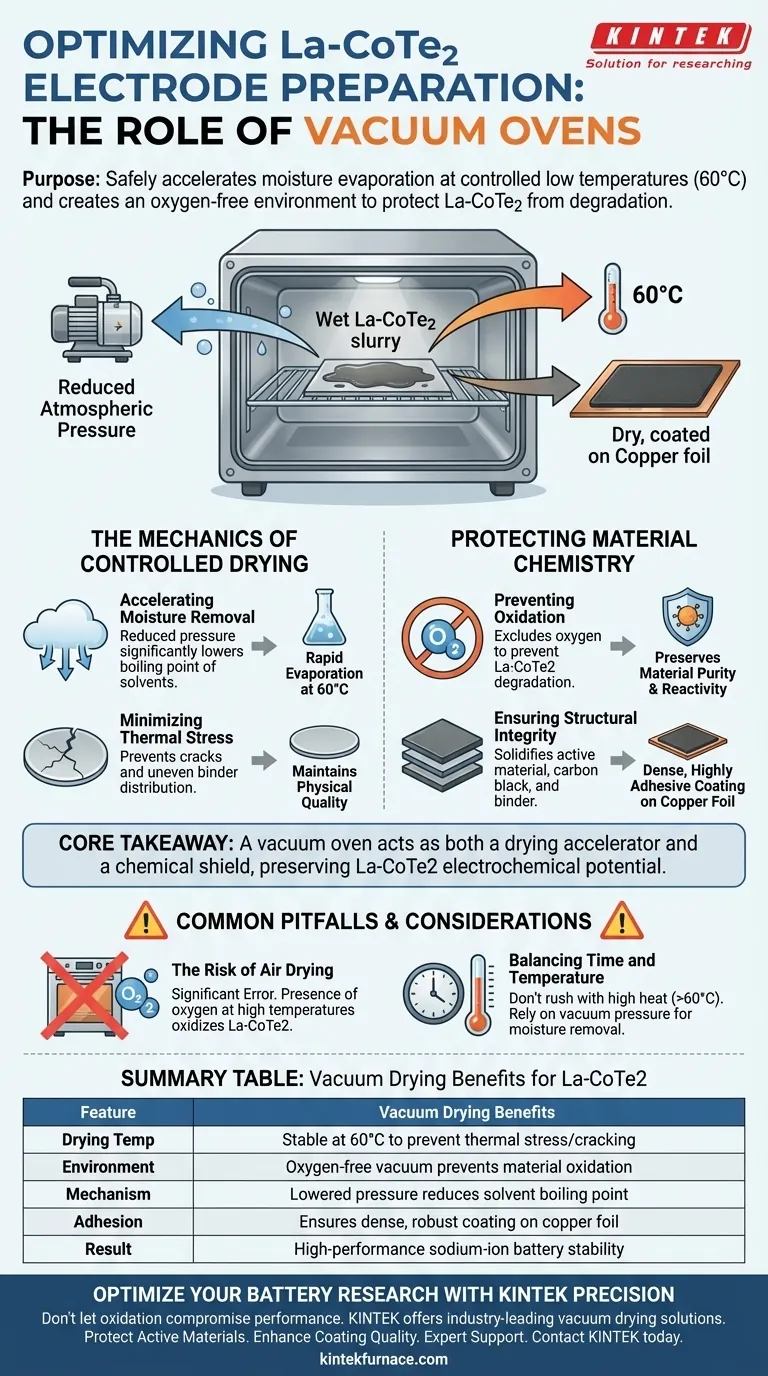
The primary purpose of using a vacuum oven is to safely accelerate the evaporation of moisture from the electrode slurry at controlled, low temperatures like 60 °C. Crucially, this creates an oxygen-free environment that prevents the active Lanthanum-doped Cobalt Telluride (La-CoTe2) from reacting with air, protecting it from chemical degradation during the drying process.
Core Takeaway A vacuum oven serves as both a drying accelerator and a chemical shield. By lowering the boiling point of moisture, it enables efficient evaporation without high heat, preserving the electrochemical potential of the La-CoTe2 material while ensuring the electrode coating adheres firmly to the current collector.

The Mechanics of Controlled Drying
Accelerating Moisture Removal
The preparation of electrode plates involves a wet slurry that must be dried completely to function. A vacuum oven reduces the atmospheric pressure surrounding the plates.
This pressure drop significantly lowers the boiling point of water and other solvents. Consequently, moisture evaporates rapidly even at a relatively low temperature of 60 °C.
Minimizing Thermal Stress
Drying at lower temperatures is essential for maintaining the physical quality of the electrode. High heat can cause cracks or uneven distribution of the binder.
By using a vacuum to drive evaporation rather than excessive heat, the process protects the delicate balance of the slurry components.
Protecting Material Chemistry
Preventing Oxidation
The most critical function of the vacuum environment is the exclusion of oxygen. Lanthanum-doped Cobalt Telluride (La-CoTe2) is sensitive to environmental factors.
If exposed to oxygen during the heating phase, the active material can degrade. The vacuum ensures the chemical composition remains pure and reactive for sodium-ion storage.
Ensuring Structural Integrity
The drying process solidifies the mixture of active material, conductive carbon black, and binder.
Proper vacuum drying results in a dense and highly adhesive coating on the copper foil current collector. This strong adhesion is vital for the mechanical stability of the battery during charge and discharge cycles.
Common Pitfalls and Considerations
The Risk of Air Drying
Attempting to dry these electrodes in a standard air oven is a significant error. Without the vacuum, the presence of oxygen at elevated temperatures will likely oxidize the La-CoTe2.
Balancing Time and Temperature
While vacuum drying accelerates the process, rushing it with temperatures significantly above 60 °C can still be detrimental. It is crucial to rely on the vacuum pressure, not just thermal energy, to remove the moisture.
Making the Right Choice for Your Goal
To ensure high-performance sodium-ion batteries, the drying protocol must be precise.
- If your primary focus is material purity: Prioritize the vacuum level to ensure zero oxygen exposure, preventing degradation of the La-CoTe2.
- If your primary focus is mechanical durability: adhere strictly to the low-temperature (60 °C) limit to ensure the coating remains dense and adheres well to the copper foil.
Summary: The vacuum oven is an indispensable tool that balances efficient moisture removal with chemical protection, ensuring your La-CoTe2 electrodes remain pure, dense, and physically robust.
Summary Table:
| Feature | Vacuum Drying Benefits for La-CoTe2 |
|---|---|
| Drying Temp | Stable at 60 °C to prevent thermal stress/cracking |
| Environment | Oxygen-free vacuum prevents material oxidation |
| Mechanism | Lowered pressure reduces solvent boiling point |
| Adhesion | Ensures dense, robust coating on copper foil |
| Result | High-performance sodium-ion battery stability |
Optimize Your Battery Research with KINTEK Precision
Don't let oxidation compromise your sodium-ion battery performance. KINTEK provides industry-leading vacuum drying solutions designed to protect sensitive materials like La-CoTe2. Backed by expert R&D and manufacturing, we offer high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all customizable to your specific laboratory requirements.
Take the next step in material integrity:
- Protect Active Materials: Ensure an oxygen-free environment.
- Enhance Coating Quality: Achieve superior adhesion and density.
- Expert Support: Benefit from our deep knowledge in high-temp lab furnaces.
Contact KINTEK today to discuss your custom furnace needs!
Visual Guide
References
- Haonan Xie, Ting Deng. Reversible Sodium Storage of CoTe2 Anode via Lanthanum Doping. DOI: 10.3390/inorganics13060207
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Hot Press Furnace Machine Heated Vacuum Press Tube Furnace
- Vacuum Heat Treat Sintering and Brazing Furnace
People Also Ask
- What is the function of a high-temperature annealing furnace for gold paste electrodes? Optimize Sensor Conductivity
- How do vacuum sintering and annealing furnaces contribute to the densification of NdFeB magnets?
- What are the key properties of vacuum brazed connections? Achieve Strong, Clean, and Hermetic Joints
- What industries commonly use vacuum chamber furnaces? Essential for Aerospace, Medical, and More
- What are the benefits of using a vacuum atmosphere in metal melting? Achieve Ultimate Purity and Control
- Why is a vacuum distillation apparatus necessary in the Kroll process? Achieving Purity in Zirconium Sponge Production
- What factors should be considered when choosing a vacuum furnace for metal heat treatment? Key Selection Criteria Explained
- Why is an industrial-grade vacuum oven essential for alumina powder? Unlock Superior Ceramic Density



















