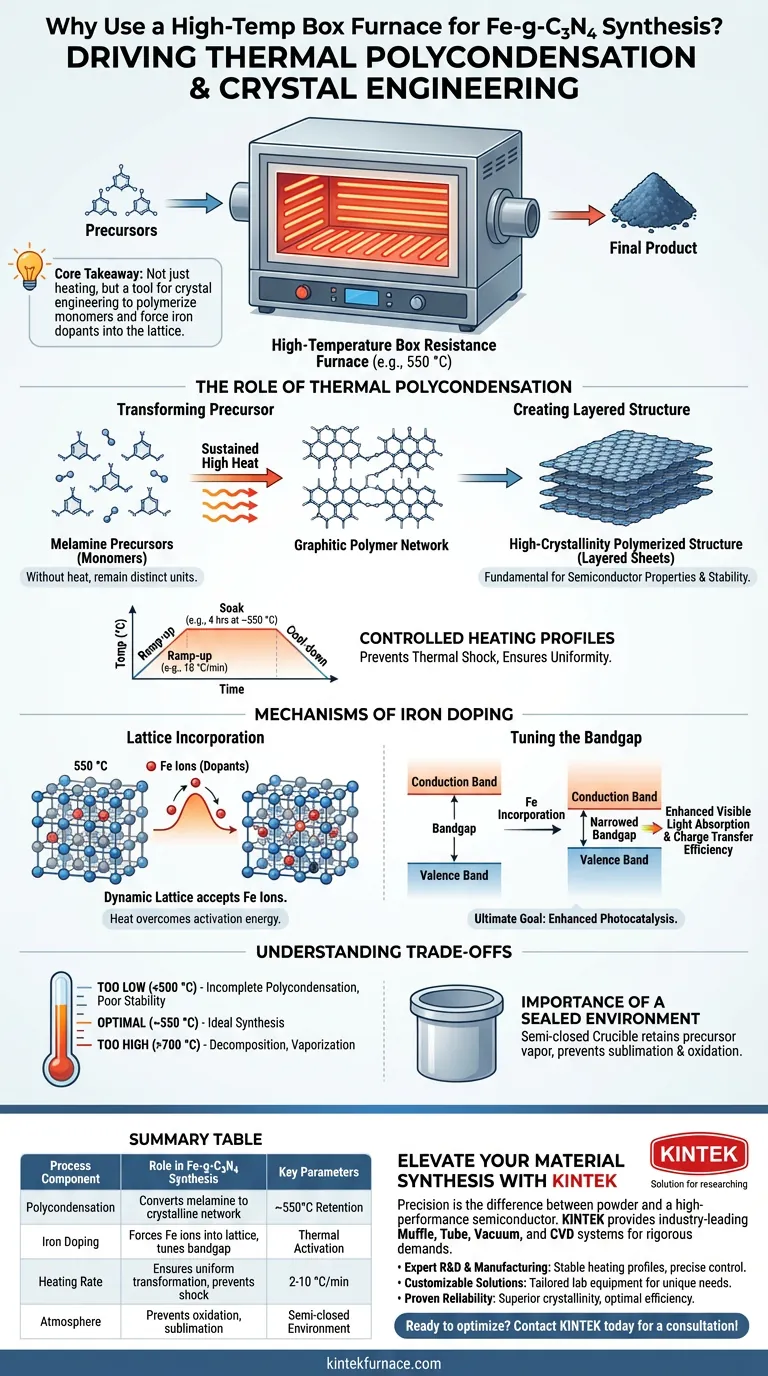
The primary purpose of a high-temperature box resistance furnace in this context is to drive thermal polycondensation. By maintaining a precise temperature, typically around 550 °C, the furnace transforms melamine precursors into a stable, layered graphitic carbon nitride (g-C3N4) structure while simultaneously facilitating the integration of iron ions into the crystal lattice.
Core Takeaway The furnace functions as a tool for crystal engineering, not just heating. It provides the specific thermal energy required to polymerize monomers into a crystalline network and force iron dopants into the lattice, which is essential for tuning the material's bandgap and enhancing its photocatalytic efficiency.

The Role of Thermal Polycondensation
Transforming the Precursor
The synthesis of graphitic carbon nitride is not a simple drying process; it is a chemical transformation.
The furnace provides the energy needed to polymerize melamine precursors (monomers). Without this sustained high heat, the precursors would remain distinct units rather than linking up to form the graphitic polymer network.
Creating the Layered Structure
The specific environment of the box furnace ensures the material develops a high-crystallinity polymerized structure.
This thermal treatment fosters the formation of the characteristic layered "graphitic" sheets. This architecture is fundamental to the material's semiconductor properties and stability.
Controlled Heating Profiles
Supplementary data indicates that precision in heating rates (e.g., 10 °C/min) is often as critical as the final temperature.
The furnace allows for a programmed ramp-up and a sustained "soak" time (often around 4 hours). This prevents thermal shock and ensures the chemical transformation is uniform throughout the sample.
Mechanisms of Iron Doping
Lattice Incorporation
The most critical function regarding the "Fe" in Fe-g-C3N4 is the incorporation of iron ions.
At 550 °C, the lattice formation is dynamic enough to accept iron ions as dopants. The furnace ensures the heat is sufficient to overcome the activation energy required for these ions to chemically bond within or between the carbon nitride layers.
Tuning the Bandgap
The ultimate goal of this thermal doping process is to alter the electronic structure of the material.
By successfully embedding iron, the process tunes the bandgap of the semiconductor. This modification directly results in enhanced absorption of visible light and improved charge transfer efficiency, making the material a more effective photocatalyst.
Understanding the Trade-offs
Temperature Sensitivity
While high heat is necessary, temperature precision is paramount.
If the temperature is too low (below ~500 °C), polycondensation will be incomplete, resulting in a material with poor stability. If the temperature is excessive (approaching 700 °C+), the carbon nitride structure effectively decomposes and vaporizes.
The Importance of a Sealed Environment
Standard box furnaces heat the air inside the chamber, which can lead to oxidation.
To counter this, the synthesis typically occurs within a protected, sealed crucible inside the furnace. This semi-closed system retains the vapor pressure of the precursors, preventing them from sublimating away before they have a chance to polymerize.
Making the Right Choice for Your Goal
When configuring your thermal treatment for Fe-g-C3N4 synthesis, consider your specific performance targets:
- If your primary focus is Structural Integrity: Prioritize a slow ramp rate (e.g., 2-5 °C/min) to ensure a defect-free, highly crystalline layered structure.
- If your primary focus is Photocatalytic Efficiency: Ensure the soak temperature reaches the full 550 °C to maximize the incorporation of iron ions for optimal bandgap narrowing.
The furnace is the gatekeeper of your material's electronic properties; precise thermal control is the only way to transition from a simple powder to a functional semiconductor.
Summary Table:
| Process Component | Role in Fe-g-C3N4 Synthesis | Key Parameters |
|---|---|---|
| Polycondensation | Converts melamine precursors into a layered crystalline network. | ~550°C Retention |
| Iron Doping | Forces iron ions into the lattice to tune the semiconductor bandgap. | Thermal Activation |
| Heating Rate | Ensures uniform chemical transformation and prevents thermal shock. | 2-10 °C/min |
| Atmosphere | Uses sealed crucibles to prevent oxidation and precursor sublimation. | Semi-closed environment |
Elevate Your Material Synthesis with KINTEK
Precision is the difference between a simple powder and a high-performance semiconductor. KINTEK provides industry-leading Muffle, Tube, Vacuum, and CVD systems designed to meet the rigorous demands of thermal polycondensation and crystal engineering.
Why choose KINTEK?
- Expert R&D & Manufacturing: Our furnaces offer the stable heating profiles (up to 10 °C/min) and precise temperature control required for iron-doped graphitic carbon nitride synthesis.
- Customizable Solutions: Whether you need a high-temperature box furnace or a specialized rotary system, we tailor our lab equipment to your unique research needs.
- Proven Reliability: Backed by years of expertise, we help researchers achieve superior crystallinity and optimal photocatalytic efficiency.
Ready to optimize your semiconductor research? Contact KINTEK today for a consultation!
Visual Guide
References
- Chien‐Yie Tsay, Shu‐Yii Wu. Fe-Doped g-C3N4/Bi2MoO6 Heterostructured Composition with Improved Visible Photocatalytic Activity for Rhodamine B Degradation. DOI: 10.3390/molecules29112631
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What is the role of a Muffle Furnace in the synthesis of PTI/LiCl? Achieve High-Crystallinity Poly(triazine imide)
- How is a laboratory high-temperature muffle furnace utilized to achieve the specific crystalline structure of LaFeO3 catalysts?
- What role does a high-temperature muffle furnace play in g-C3N4 catalyst synthesis? Precision Pyrolysis Solutions
- How do box type resistance furnaces contribute to catalytic material preparation? Unlock Precision in Catalyst Synthesis
- What temperature considerations are important for muffle furnaces? Optimize Performance and Longevity
- What is the function of an industrial muffle furnace in stir casting? Enhance Aluminum Matrix Composite Production
- What is a muffle furnace and what is its primary use? Ensure Purity in High-Temperature Processes
- What is the role of a muffle furnace in the preparation of CeO2? Engineer High-Purity Single-Atom Catalyst Supports



















