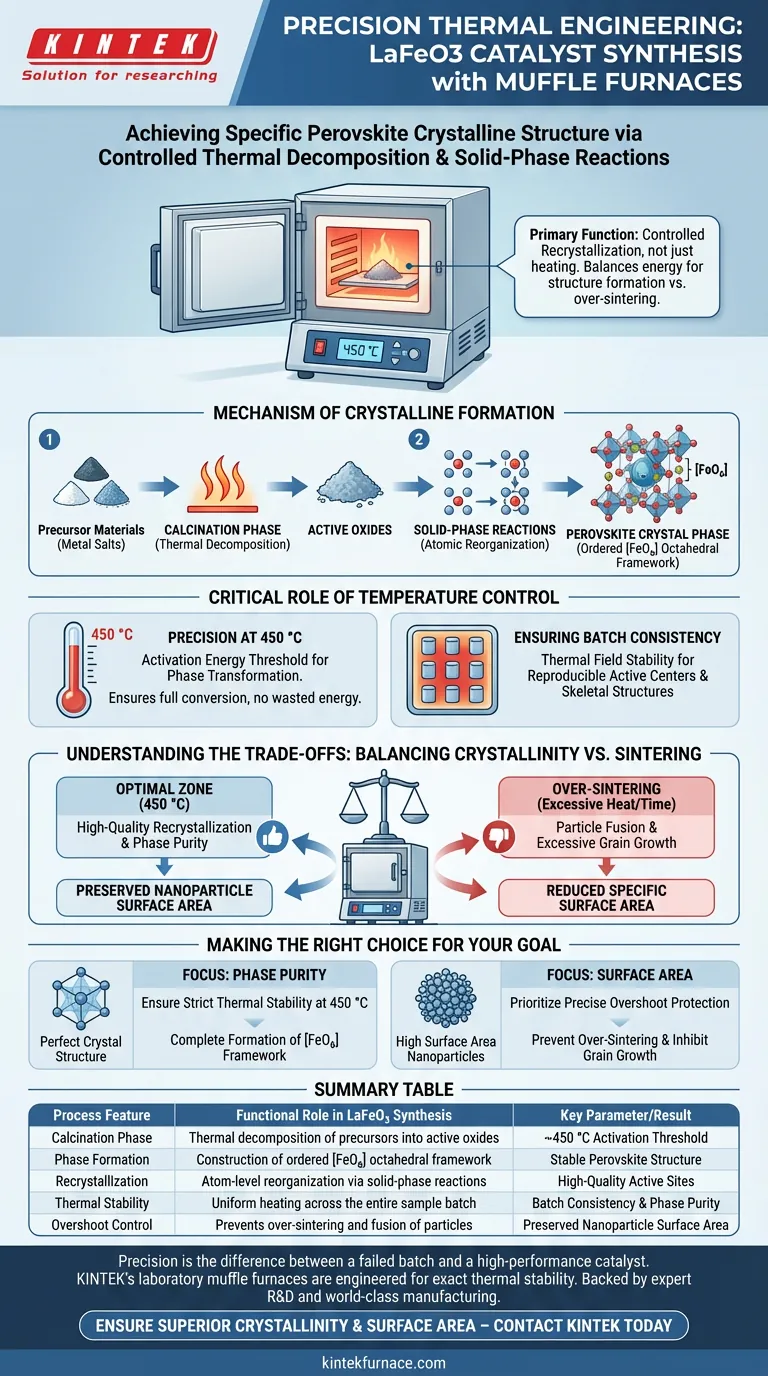
A laboratory high-temperature muffle furnace achieves the specific crystalline structure of LaFeO3 catalysts by facilitating precise thermal decomposition and solid-phase reactions. Specifically, it subjects precursors to a controlled calcination process, typically around 450 °C. This thermal environment converts the material into a perovskite crystal phase characterized by an ordered [FeO6] octahedral framework.
The furnace’s primary function is not just heating, but controlled recrystallization. It balances the energy required to form the perovskite structure against the risk of over-sintering, ensuring high-quality active sites without excessive grain growth.
The Mechanism of Crystalline Formation
Thermal Decomposition and Solid-Phase Reactions
The formation of LaFeO3 is driven by a calcination phase, where the muffle furnace applies heat to decompose precursor materials.
During this stage, metal salt precursors are broken down into active oxides. This triggers solid-phase reactions, forcing the material to reorganize at the atomic level rather than simply melting or drying.
Constructing the Perovskite Structure
The specific goal of this thermal treatment is the creation of a perovskite crystal phase.
The muffle furnace provides the sustained thermal energy required to arrange the atoms into a specific geometry. For LaFeO3, this results in the formation of an ordered [FeO6] octahedral framework, which is critical for the catalyst's final performance.
The Critical Role of Temperature Control
Precision at 450 °C
According to standard protocols for this material, the furnace is often set to a specific target, such as 450 °C.
Maintaining this exact temperature is vital because it represents the activation energy threshold for the phase transformation. It ensures the precursors fully convert into the desired crystalline form without wasting energy or damaging the material.
Ensuring Batch Consistency
A key advantage of a high-quality laboratory muffle furnace is its thermal field stability.
This stability ensures that every part of the sample receives the same thermal history. This consistency allows for the reproducible formation of active centers and skeletal structures across different batches of the catalyst.
Understanding the Trade-offs
Balancing Crystallinity vs. Sintering
The most critical challenge in preparing LaFeO3 is finding the "Goldilocks" zone of thermal energy.
You need high temperatures to ensure high-quality recrystallization and phase purity. However, excessive heat or prolonged exposure can lead to over-sintering, where the particles fuse together undesirably.
The Risk of Grain Growth
If the muffle furnace lacks precision or overshoots the target temperature, it causes excessive grain growth.
Large grains reduce the specific surface area of the material. By strictly controlling the temperature at 450 °C, the furnace prevents this growth, preserving the nanoparticles and ensuring the active sites remain accessible.
Making the Right Choice for Your Goal
To optimize your LaFeO3 catalyst preparation, align your furnace usage with your specific objectives:
- If your primary focus is Phase Purity: Ensure your furnace can maintain strict thermal stability at 450 °C to guarantee the complete formation of the [FeO6] octahedral framework.
- If your primary focus is Surface Area: Prioritize precise overshoot protection to prevent over-sintering and inhibit excessive grain growth during recrystallization.
Success in catalyst synthesis relies on treating the muffle furnace as a precision instrument for crystal engineering, not merely a source of heat.
Summary Table:
| Process Feature | Functional Role in LaFeO3 Synthesis | Key Parameter/Result |
|---|---|---|
| Calcination Phase | Thermal decomposition of precursors into active oxides | ~450 °C Activation Threshold |
| Phase Formation | Construction of ordered [FeO6] octahedral framework | Stable Perovskite Structure |
| Recrystallization | Atom-level reorganization via solid-phase reactions | High-Quality Active Sites |
| Thermal Stability | Uniform heating across the entire sample batch | Batch Consistency & Phase Purity |
| Overshoot Control | Prevents over-sintering and fusion of particles | Preserved Nanoparticle Surface Area |
Precision is the difference between a failed batch and a high-performance catalyst. KINTEK’s laboratory muffle furnaces are engineered for the exact thermal stability required for complex phase transformations like LaFeO3 synthesis. Backed by expert R&D and world-class manufacturing, KINTEK offers Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique research needs. Ensure your catalysts maintain superior crystallinity and surface area—Contact KINTEK today to find your ideal high-temperature solution.
Visual Guide
References
- Tian Guo, Fei Wei. Upgrading CO2 to sustainable aromatics via perovskite-mediated tandem catalysis. DOI: 10.1038/s41467-024-47270-z
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- What role does a Muffle Furnace play in processing steel and soil samples for cobalt extraction? Essential Lab Guide
- How is a muffle furnace utilized for defect engineering in delta-MnO2? Precision Thermal Treatment for Optimal Defects
- What role do box type electric furnaces play in the glass industry? Essential for Precise R&D and Testing
- How is a high-temperature box resistance furnace utilized for ZK51A T1 heat treatment? Optimize Mg Alloy Hardening
- How does an industrial electric box furnace maintain sample alignment? Ensure Precision in High-Throughput Calcination
- What is the role of a laboratory muffle furnace in ilmenite pretreatment? Optimize Thermal Activation at 950 °C
- How is a laboratory high-temperature muffle furnace utilized in g-C3N4 synthesis? Optimize Your Thermal Polycondensation
- What is the primary function of a high-temperature muffle furnace in ilmenite smelting? Enhance Carbothermic Efficiency



















