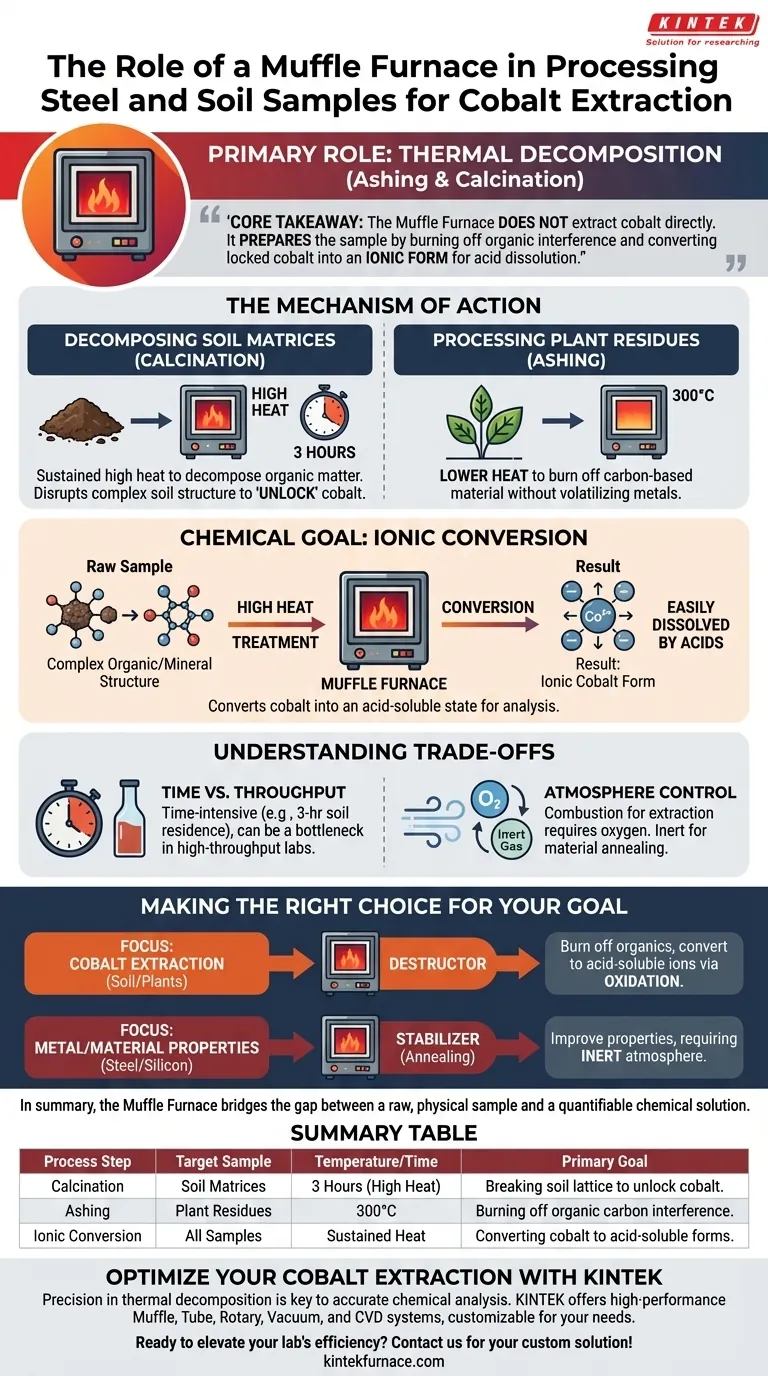
A Muffle Furnace serves as the primary instrument for thermal decomposition (ashing and calcination) during sample preparation. In the context of cobalt extraction, its specific role is to eliminate organic interference and break down complex matrices in soil and plant samples, rendering the cobalt chemically accessible for analysis.
(Note: While Muffle Furnaces are commonly used for heat-treating metals, the primary reference for this specific cobalt extraction process specifies plant samples rather than steel. The details below reflect this source material.)
The Core Takeaway The Muffle Furnace does not extract cobalt directly; rather, it prepares the sample for extraction. By subjecting samples to controlled high heat, it burns off organic "noise" and converts locked cobalt into an ionic form, allowing acids to easily dissolve it for accurate measurement.

The Mechanism of Action: Ashing and Calcination
The Muffle Furnace acts as a high-precision incinerator. Its goal is to strip away the physical structure of the sample—whether biological or geological—to isolate the chemical elements within.
Decomposing Soil Matrices
For soil samples, the furnace provides a sustained, high-temperature environment required for calcination.
The process typically involves maintaining high heat for 3 hours. This duration ensures the complete decomposition of organic matter found in the soil.
More importantly, this heat disrupts the complex structural matrix of the soil. This thermal shock is necessary to "unlock" cobalt from the soil's physical lattice.
Processing Plant Residues
When dealing with plant-based samples (often analyzed alongside soil in environmental studies), the furnace performs ashing.
The furnace is set to 300°C to ash dry residues. This lower temperature (compared to some industrial treatments) is sufficient to burn off carbon-based plant material without volatilizing the target metals.
The Chemical Goal: Ionic Conversion
The ultimate objective of these thermal processes is chemical conversion.
Raw samples often contain cobalt bound in complex organic or mineral structures that liquid acids cannot penetrate.
The heat treatment converts this cobalt into an ionic form. Once in this state, the cobalt can be easily dissolved by acids in subsequent steps, making it ready for spectral analysis or extraction.
Understanding the Trade-offs
While Muffle Furnaces are essential for accuracy, they introduce specific variables that must be managed.
Time vs. Throughput
The process is time-intensive. With soil samples requiring a 3-hour residence time, the furnace can become a bottleneck in high-throughput laboratories.
Atmosphere Control
As noted in broader industrial applications, Muffle Furnaces often utilize controlled atmospheres (like inert gas or oxygen-free environments).
However, for ashing and calcination, the presence of oxygen is often required to facilitate combustion. Operators must ensure the specific atmosphere matches the goal: combustion for extraction vs. oxidation prevention for material annealing.
Making the Right Choice for Your Goal
Depending on your specific analytical or processing needs, the role of the furnace shifts.
- If your primary focus is Cobalt Extraction (Soil/Plants): You are using the furnace as a destructor to burn off organics and convert metals into acid-soluble ions using oxidation.
- If your primary focus is Metal/Material Properties (Steel/Silicon): You are using the furnace as a stabilizer (annealing) to improve crystallinity and conductivity, often requiring an inert (oxygen-free) atmosphere to prevent damage.
In summary, the Muffle Furnace bridges the gap between a raw, physical sample and a quantifiable chemical solution.
Summary Table:
| Process Step | Target Sample | Temperature / Time | Primary Goal |
|---|---|---|---|
| Calcination | Soil Matrices | 3 Hours (High Heat) | Breaking soil lattice to unlock cobalt |
| Ashing | Plant Residues | 300°C | Burning off organic carbon interference |
| Ionic Conversion | All Samples | Sustained Heat | Converting cobalt to acid-soluble forms |
Optimize Your Cobalt Extraction with KINTEK
Precision in thermal decomposition is the key to accurate chemical analysis. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique laboratory or industrial high-temp furnace needs. Whether you are performing critical ashing for soil samples or complex heat treatments for steel, our equipment ensures the thermal stability and atmospheric control you require.
Ready to elevate your lab's efficiency? Contact us today to find your custom solution!
Visual Guide
References
- Kerim A. Kuliyev, Naiba N Efendiyeva. Spectroscopic Study of Complex Formation of Cobalt (Ii) with 2,6-Mercapto-4-Sec-Butylphenol and Heterocyclic Amines. DOI: 10.64030/3065-906x.02.01.04
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the significance of using a high-temperature muffle furnace for Co3O4 nanotube stabilization? Ensure Robustness & Chemical Resilience.
- What is the primary role of a muffle furnace in the calcination of Pt-xWO3/SiO2? Optimize Catalyst Phase-Engineering
- Why is a box muffle furnace required for In2O3 nanofibers? Expert Synthesis & Pre-Oxidation Guide
- How is a muffle furnace utilized in research and medical laboratories? Essential for Contaminant-Free High-Temperature Processing
- What should be considered when purchasing a box type electric furnace? Key Factors for Optimal Thermal Processing
- What are the temperature capabilities of drying ovens compared to muffle furnaces? Choose the Right Tool for Your Lab
- How is a Muffle furnace used in environmental treatment? Essential for Waste Analysis and Small-Scale Incineration
- How is an industrial-grade ashing furnace utilized in 3D-printed bioactive glass? Master Debinding & Sintering



















