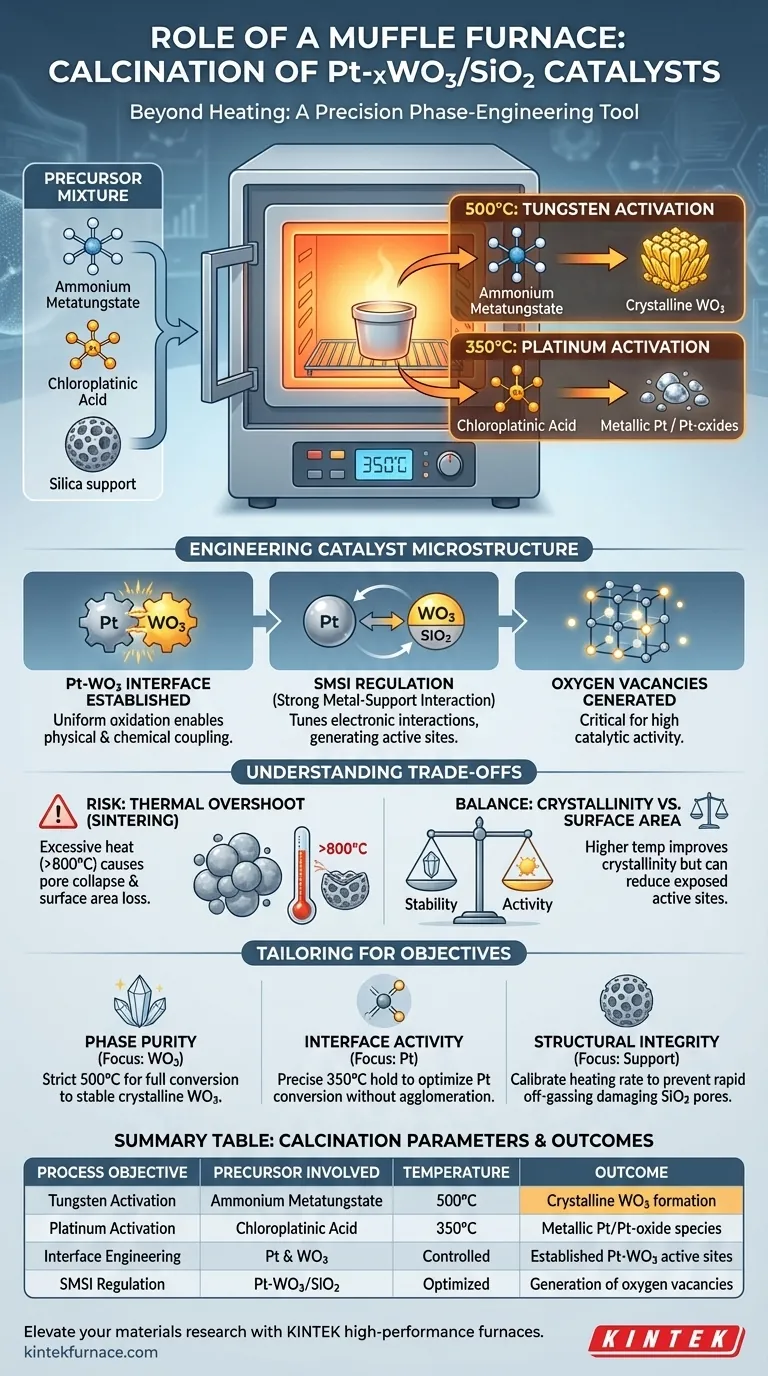
The primary role of a muffle furnace in this context is to provide a precision-controlled thermal environment that drives the chemical decomposition of specific precursors into active catalyst phases. For Pt-xWO3/SiO2 catalysts, this involves converting ammonium metatungstate into crystalline tungsten trioxide (WO3) at 500°C and transforming chloroplatinic acid into metallic platinum or platinum oxides at 350°C.
The muffle furnace is not merely a heating device; it is a phase-engineering tool. It is essential for establishing the critical Pt-WO3 interface, regulating strong metal-support interactions (SMSI), and generating the oxygen vacancies required for high catalytic activity.

Engineering the Catalyst Microstructure
Precursor Decomposition and Phase Transformation
The fundamental function of the muffle furnace is to facilitate the complete thermal decomposition of chemical precursors.
For the tungsten component, the furnace must maintain 500°C to break down ammonium metatungstate. This specific temperature regime ensures the formation of stable, crystalline tungsten trioxide (WO3) phases on the silica support.
Activation of Platinum Species
Distinct from the tungsten activation, the platinum component requires a different thermal treatment profile.
Heating the material at 350°C allows for the controlled conversion of chloroplatinic acid precursors. This step effectively removes chloride ligands, resulting in the formation of metallic platinum or platinum oxide species.
Establishing the Active Interface
The most critical outcome of this thermal processing is the creation of the Pt-WO3 interface.
By providing a uniform oxidation environment, the furnace enables the physical and chemical coupling of the platinum and tungsten species. This interaction is responsible for the unique electronic properties of the catalyst.
Regulating Electronic Interactions
The calcination process directly influences the Strong Metal-Support Interaction (SMSI).
Proper thermal treatment in the muffle furnace tunes how strongly the platinum interacts with the tungsten-modified support. This regulation is vital for creating oxygen vacancies, which serve as active sites for subsequent chemical reactions.
Understanding the Trade-offs
The Risk of Thermal Overshoot (Sintering)
While high temperatures are necessary for decomposition, excessive heat is detrimental.
If the muffle furnace temperature exceeds optimal limits (e.g., reaching 800°C as noted in general catalyst synthesis), it can lead to severe sintering. This causes the collapse of the pore structure and a significant reduction in the specific surface area.
Balancing Crystallinity and Surface Area
There is an inherent trade-off between forming stable crystals and maintaining a high surface area.
Higher temperatures generally improve the crystallinity of the WO3 and Pt phases, which adds stability. However, aggressive heating can reduce the number of exposed surface active sites, diminishing overall performance.
Making the Right Choice for Your Goal
To maximize the efficacy of your Pt-xWO3/SiO2 catalyst, you must tailor the muffle furnace parameters to your specific objectives.
- If your primary focus is Phase Purity: Adhere strictly to the 500°C setpoint to ensure the ammonium metatungstate is fully converted into the stable WO3 crystalline phase.
- If your primary focus is Interface Activity: Prioritize precise temperature holding at 350°C to optimize the Pt precursor conversion without inducing premature agglomeration of the metal particles.
- If your primary focus is Structural Integrity: Calibrate the heating rate carefully to prevent rapid off-gassing of ligands, which can damage the pore structure of the SiO2 support.
Success relies on treating the muffle furnace as a precision instrument for chemical synthesis, rather than a simple drying oven.
Summary Table:
| Process Objective | Precursor Involved | Temperature | Outcome |
|---|---|---|---|
| Tungsten Activation | Ammonium Metatungstate | 500°C | Crystalline WO3 formation |
| Platinum Activation | Chloroplatinic Acid | 350°C | Metallic Pt/Pt-oxide species |
| Interface Engineering | Pt & WO3 | Controlled | Established Pt-WO3 active sites |
| SMSI Regulation | Pt-WO3/SiO2 | Optimized | Generation of oxygen vacancies |
Precision is the difference between a failed batch and a high-activity catalyst. KINTEK provides high-performance Muffle, Tube, and Vacuum furnaces specifically designed for phase-engineering and sensitive calcination processes. Backed by expert R&D and manufacturing, our systems are fully customizable to your unique catalyst research needs. Elevate your materials research—contact KINTEK today for a custom solution.
Visual Guide
References
- Wanru Yan, Yu Tang. Investigation on Pt-WO3 Catalytic Interface for the Hydrodeoxygenation of Anisole. DOI: 10.3390/catal15090859
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why use a high-temp box resistance furnace for Ca2.5Ag0.3Sm0.2Co4O9 sintering? Ensure Phase Purity and Alignment
- Why use a high-temp sintering furnace at 750°C for silver nanoparticles? Achieve purity and stability.
- What thermal processes can be performed using Box Furnaces? Unlock Versatile Heat Treatment Solutions
- What types of heating elements are used in muffle furnaces and their temperature ranges? Choose the Right Element for Your Lab
- What maintenance procedures are recommended for muffle furnaces? Ensure Accuracy and Safety in Your Lab
- How does a muffle furnace contribute to the post-processing of SnO2? Engineering Superior Nanoparticle Crystallinity
- What function does a muffle furnace perform in Yttrium Oxide synthesis? Master Polycrystalline Active Layer Formation
- How are box type electric furnaces applied in electronic component manufacturing? Unlock Precision Thermal Processing



















