A high-temperature sintering furnace operating at 750°C is primarily used to purify the nanoparticle surface and stabilize its internal crystal structure. This thermal treatment triggers the decomposition of residual organic materials and biological impurities, ensuring the final silver nanoparticles are chemically pure and structurally sound.
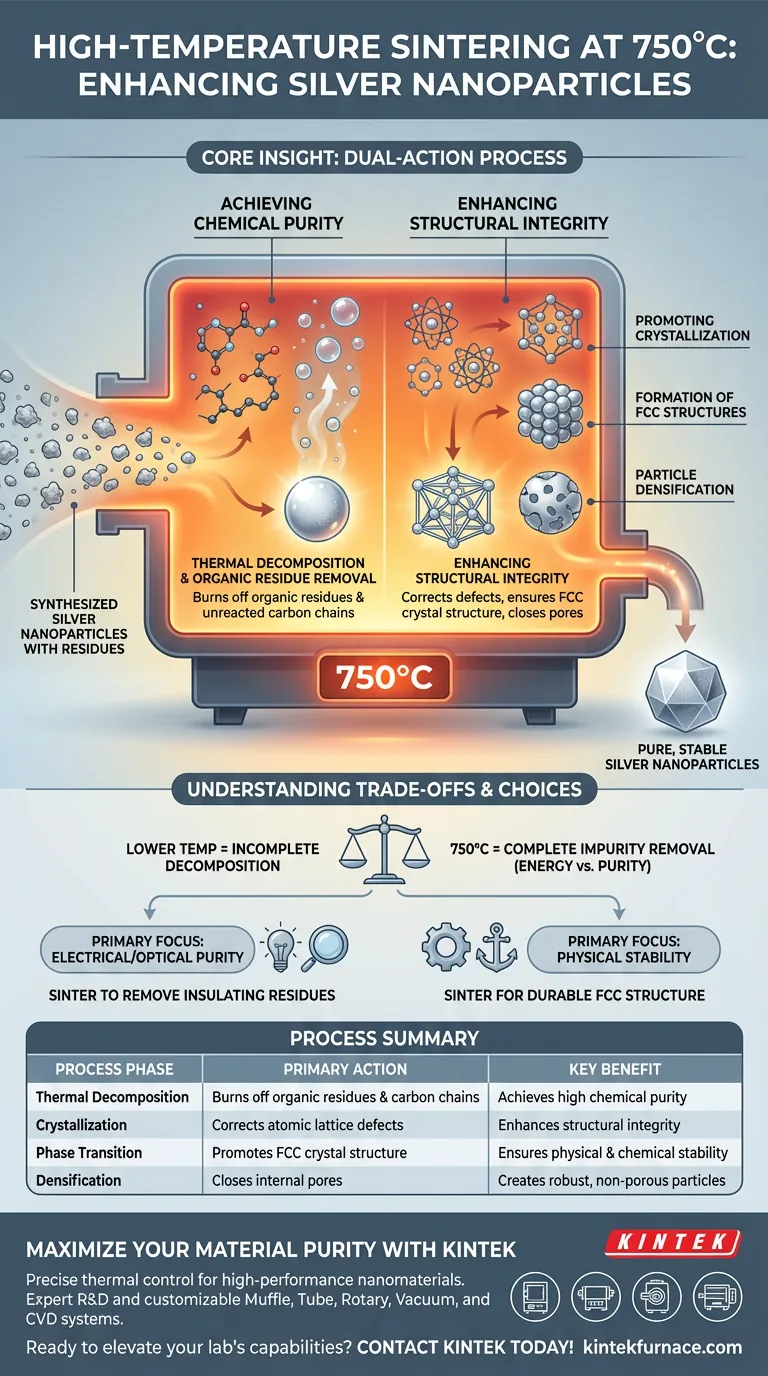
Core Insight: Sintering at this specific temperature is a dual-action process: it acts as a cleaning agent by burning off carbon-based contaminants and as a structural architect by forcing the silver atoms into a stable, dense arrangement.
Achieving Chemical Purity
Removal of Organic Residues
During the chemical synthesis of silver nanoparticles, various precursors and stabilizing agents are used.
A key function of the 750°C environment is to induce thermal decomposition. This effectively burns off organic residues and biological impurities that adhere to the surface of the nanoparticles during the initial preparation.
Elimination of By-products
The high thermal energy targets specific synthesis by-products.
Specifically, it eliminates unreacted carbon chains. Removing these contaminants is essential to prevent them from interfering with the material's final electrical or optical properties.
Enhancing Structural Integrity
Promoting Crystallization
Beyond cleaning, the heat serves as activation energy for the silver atoms themselves.
The sintering process improves the overall crystallization of the nanoparticles. It corrects defects in the atomic lattice that may have formed during the rapid precipitation phases of synthesis.
Formation of Face-Centered Cubic Structures
The 750°C threshold promotes a specific, highly stable atomic arrangement.
It drives the silver to adopt a face-centered cubic (FCC) crystal structure. This specific phase is critical for ensuring the material exhibits the expected physical and chemical stability inherent to metallic silver.
Particle Densification
Finally, the heat regulates the physical density of the material.
The process facilitates particle densification, closing internal pores and ensuring the nanoparticles are solid and robust rather than porous or fragile.
Understanding the Process Trade-offs
The Necessity of High Thermal Energy
While lower temperatures might induce some drying, they often fail to fully decompose complex organic chains.
The specific choice of 750°C is a trade-off favoring complete impurity removal over energy conservation. A lower temperature would likely leave carbon residues that degrade the nanoparticle's performance.
Precision vs. Aggregation
While the primary goal is densification, thermal processing must be carefully controlled.
As noted in broader thermal processing contexts, high heat provides activation energy. However, it must be maintained at a constant temperature to ensure uniformity; fluctuations could lead to uneven crystal growth or incomplete phase transformation.
Making the Right Choice for Your Goal
When determining if this specific post-processing step is required for your material, consider your end-use requirements:
- If your primary focus is electrical or optical purity: You must utilize high-temperature sintering to ensure complete removal of insulating organic residues and carbon chains.
- If your primary focus is physical stability: You should rely on this process to force the transition into a durable face-centered cubic crystal structure.
High-temperature sintering is the definitive step that transforms synthesized raw precipitate into high-performance, functional silver nanomaterials.
Summary Table:
| Process Phase | Primary Action | Key Benefit |
|---|---|---|
| Thermal Decomposition | Burns off organic residues & carbon chains | Achieves high chemical purity |
| Crystallization | Corrects atomic lattice defects | Enhances structural integrity |
| Phase Transition | Promotes FCC crystal structure | Ensures physical & chemical stability |
| Densification | Closes internal pores | Creates robust, non-porous particles |
Maximize Your Material Purity with KINTEK
Precise thermal control is the difference between a contaminated sample and a high-performance nanomaterial. KINTEK provides industry-leading high-temperature sintering solutions tailored for advanced research and industrial applications.
Backed by expert R&D and manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to meet your specific temperature thresholds and atmosphere requirements. Whether you are perfecting silver nanoparticle synthesis or developing next-generation ceramics, our lab high-temp furnaces ensure the thermal stability and uniform heat distribution you need.
Ready to elevate your lab's capabilities? Contact KINTEK today to find your perfect furnace solution!
Visual Guide
References
- Muneeb Irshad, Martin Motola. Harnessing bio-based chelating agents for sustainable synthesis of AgNPs: Evaluating their inherent attributes and antimicrobial potency in conjunction with honey. DOI: 10.1016/j.heliyon.2024.e31424
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Spark Plasma Sintering SPS Furnace
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 9MPa Air Pressure Vacuum Heat Treat and Sintering Furnace
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- Why are muffle furnaces important in laboratories? Essential for Contamination-Free High-Temperature Processing
- How to keep samples in muffle furnace? A Step-by-Step Guide for Safe & Accurate Results
- How is a muffle furnace utilized to determine the thermal stability of NaA zeolite? Expert Stress-Test Analysis
- What is the function of a laboratory muffle furnace in treating LNMO precursors? Ensure High-Purity Material Synthesis
- How do high-temperature muffle furnaces and ceramic crucibles ensure accuracy? Achieve Precise Alloy Oxidation Data
- What types of facilities typically use box furnaces? Essential for Labs and Small-Scale Production
- What precautions apply when opening the furnace door at high temperatures? Ensure Safety and Prevent Damage
- What role does a high-temperature box-type resistance furnace play in solar cell electrode processing? Master Sintering



















