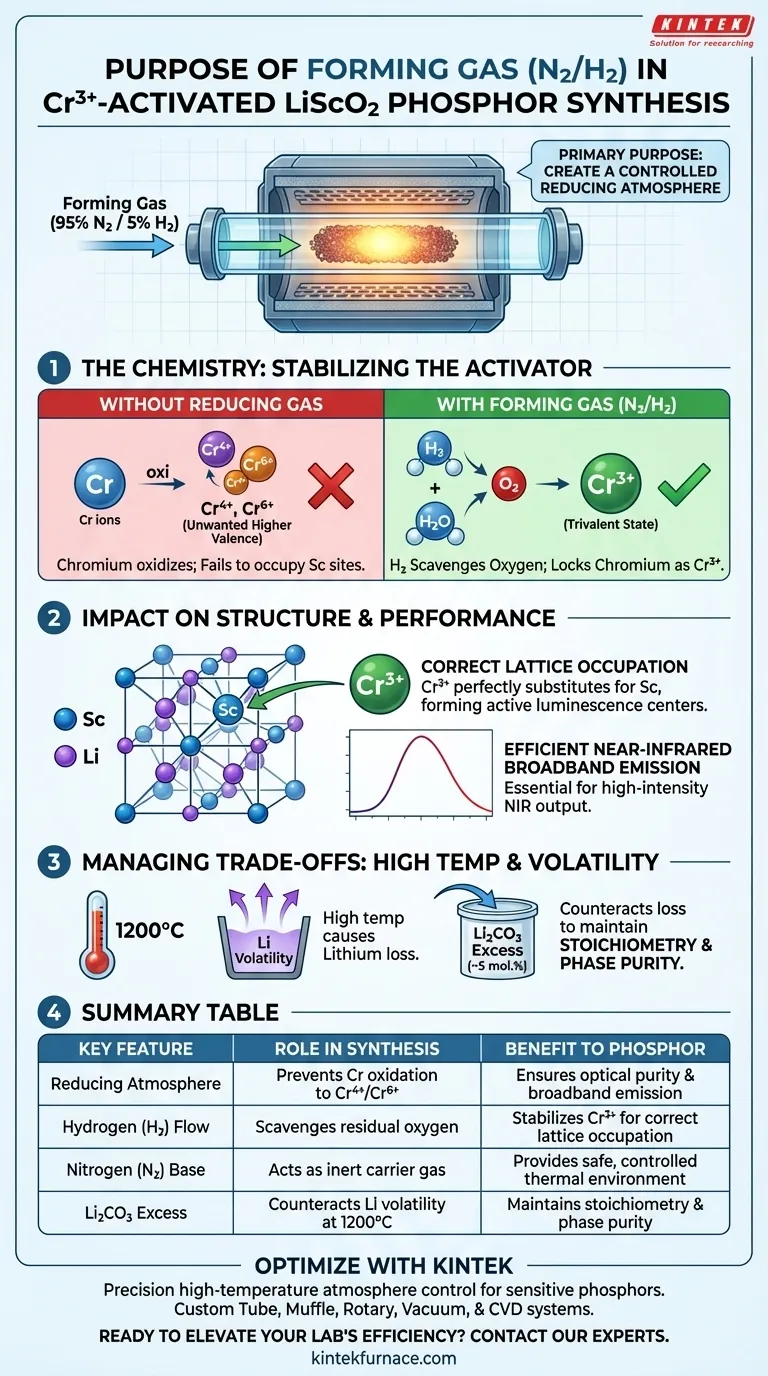
The primary purpose of using forming gas (specifically a Nitrogen/Hydrogen mix) is to create a controlled reducing atmosphere within the tube furnace. This environment is essential to chemically stabilize the Chromium activator during the high-temperature heat treatment, preventing it from reacting with oxygen to form unwanted higher valence states.
The forming gas acts as a chemical shield, ensuring the Chromium dopant remains in the trivalent state (Cr3+). This specific valence state is the only one capable of correctly occupying Scandium sites in the lattice, which is the fundamental requirement for achieving efficient near-infrared broadband emission.

The Chemistry of the Reducing Atmosphere
Preventing Unwanted Oxidation
During high-temperature synthesis, transition metals like Chromium are highly susceptible to oxidation.
Without a reducing agent, Chromium would naturally oxidize into higher valence states, specifically tetravalent (Cr4+) or hexavalent (Cr6+) ions.
Stabilizing the Trivalent State
The hydrogen component (typically 5%) in the forming gas actively scavenges residual oxygen.
This reaction forces the environment to remain reducing, locking the Chromium atoms in the critical trivalent (Cr3+) state required for this specific phosphor.
Impact on Structure and Performance
Correct Lattice Site Occupation
For the LiScO2 phosphor to function, the activator must integrate perfectly into the crystal structure.
Because Cr3+ has a specific ionic radius and charge, it is chemically suited to substitute for Scandium (Sc) ions within the host lattice.
If Chromium were allowed to oxidize to Cr4+ or Cr6+, this substitution would fail, leading to lattice defects rather than active luminescence centers.
Ensuring Optical Efficiency
The luminescence properties of the material are directly tied to the specific electronic environment of the Cr3+ ion.
By maintaining the Cr3+ state via the forming gas, you ensure the material creates stable, high-intensity near-infrared broadband emission.
Understanding the Trade-offs
High Temperature vs. Material Volatility
While high temperatures (around 1200°C) are necessary to facilitate the Cr3+ substitution, they introduce side effects that the gas alone cannot fix.
Specifically, Lithium is highly volatile at these temperatures and tends to evaporate from the material.
Managing Stoichiometry
The reducing atmosphere protects the Chromium, but it does not prevent Lithium loss.
To counter this, the synthesis requires adding approximately 5 mol.% excess Lithium Carbonate to the starting mixture.
This pre-compensation ensures the final product maintains the correct stoichiometric ratio, avoiding secondary phases that could degrade the purity guarded by the forming gas.
Making the Right Choice for Your Goal
To achieve high-quality LiScO2:Cr3+ phosphors, you must balance chemical protection with stoichiometric compensation.
- If your primary focus is Optical Purity: Ensure a consistent flow of forming gas (5% H2) to strictly prevent the formation of Cr4+ or Cr6+ species that kill luminescence.
- If your primary focus is Phase Purity: Combine the reducing atmosphere with a 5 mol.% excess of Lithium Carbonate to compensate for volatilization at 1200°C.
Mastering the atmosphere controls the activator's valence, while mastering the stoichiometry controls the host lattice's integrity.
Summary Table:
| Key Feature | Role in Synthesis | Benefit to Phosphor |
|---|---|---|
| Reducing Atmosphere | Prevents Chromium oxidation to Cr4+ or Cr6+ | Ensures optical purity and broadband emission |
| Hydrogen (H2) Flow | Scavenges residual oxygen in the furnace | Stabilizes Cr3+ ions for correct lattice occupation |
| Nitrogen (N2) Base | Acts as an inert carrier gas | Provides a safe, controlled thermal environment |
| Li2CO3 Excess | Counteracts lithium volatility at 1200°C | Maintains stoichiometry and phase purity |
Optimize Your Material Synthesis with KINTEK
Precision is paramount when managing high-temperature atmospheres for sensitive phosphors. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems, all fully customizable to meet your unique research needs.
Whether you require precise forming gas control to stabilize activators or advanced thermal uniformity to manage material volatility, our laboratory furnaces provide the reliability your innovations deserve.
Ready to elevate your lab's efficiency? Contact our experts today to find your custom furnace solution!
Visual Guide
References
- Leoni Frehmeyer, Thomas Jüstel. On the optimisation of the broadband NIR emitter LiScO2:Cr3+. DOI: 10.6001/chemija.2025.36.2.5
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
People Also Ask
- What role does a vacuum tube furnace play in the 600°C high-temperature annealing of Pd/TaTiNbZr/Ta multilayer membranes?
- Why are near alpha-titanium alloy ingots often sealed in quartz tubes? Unlock Superior Purity and Microstructure
- What is the purpose of a stepper motor equipped with a 100:1 reducer in a tube furnace? Achieve Precision Control
- What is the primary function of a tube furnace in the pyrolysis of biomass? Achieve Precision in Material Research
- Why is high-purity nitrogen gas introduced into a vacuum tube furnace during cooling? Optimize Your Nitriding Process
- What is the function of a tube reduction furnace? Enhance Ru@PG Catalysts with Ar/H2 Precision
- Why is a high-temperature tube furnace required for MoS2 and WS2 thin films? Achieve 2H Crystalline Phase Excellence
- What technological requirements affect tube furnace design? Key Factors for Optimal Performance



















