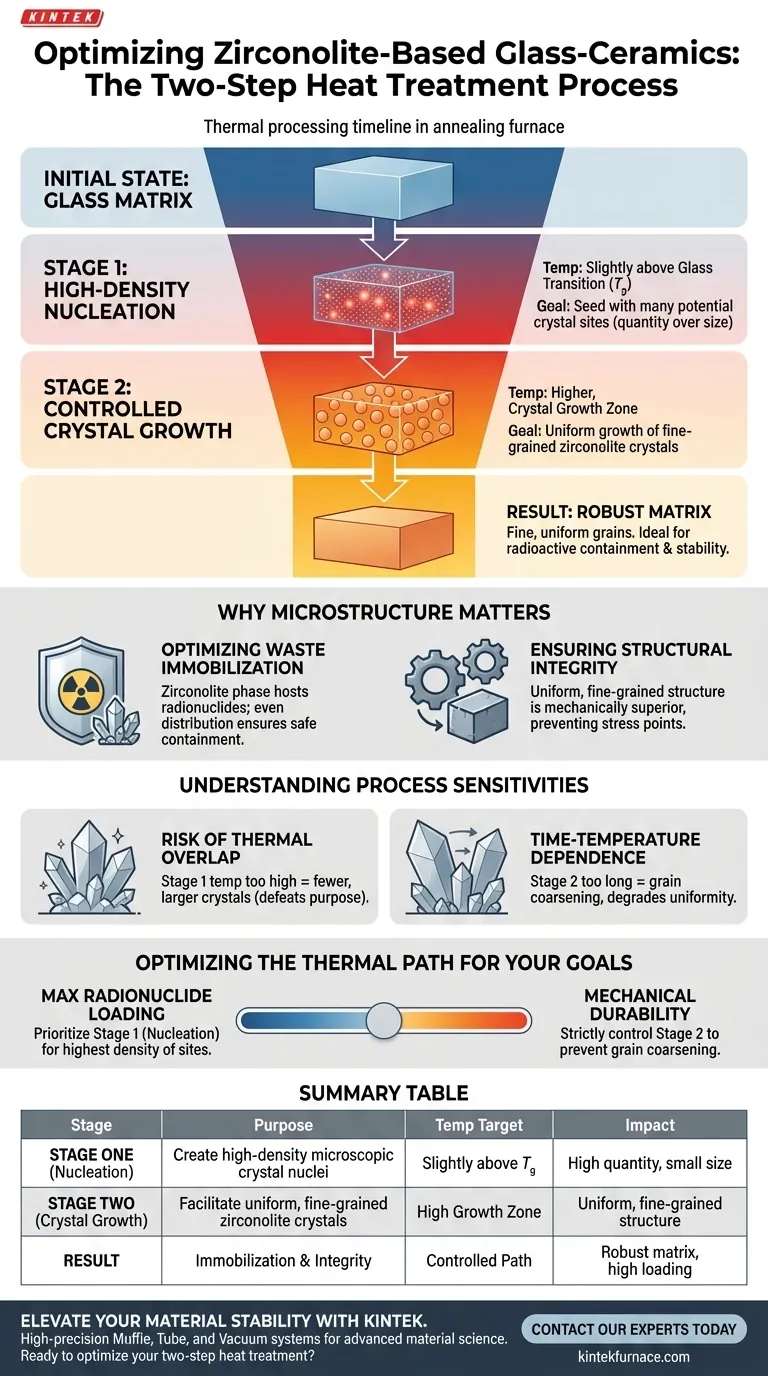
The primary purpose of the two-step heat treatment process is to decouple the nucleation mechanism from the crystal growth mechanism, allowing for precise engineering of the material's microstructure. The first step involves holding the temperature slightly above the glass transition temperature ($T_g$) to induce high-density nucleation. The second step utilizes higher temperatures to facilitate the uniform growth of zirconolite crystals, creating a robust matrix for radioactive containment.
Ideally, crystallization requires a balance between creating new crystal centers and growing existing ones. By separating these into two distinct thermal stages, this process ensures the final material is composed of fine, uniform grains rather than large, irregular crystals that could compromise stability.

The Mechanics of the Two-Step Process
Stage One: High-Density Nucleation
The first phase of the heat treatment is strictly controlled relative to the glass transition temperature ($T_g$).
By holding the matrix at a temperature just above $T_g$, the process encourages the formation of a vast number of microscopic crystal nuclei.
This stage is not about size; it is about quantity. The goal is to seed the glass matrix with as many potential crystal sites as possible without allowing them to expand immediately.
Stage Two: Controlled Crystal Growth
Once the nuclei density is established, the temperature is raised to the crystal growth zone.
In this second stage, the previously formed nuclei begin to grow into distinct zirconolite crystal grains.
Because the nuclei were formed in high density during the first step, the growth in the second step results in a fine-grained microstructure rather than a few large, isolated crystals.
Why Microstructure Matters for Containment
Optimizing Waste Immobilization
The ultimate goal of using zirconolite-based glass-ceramics is to safely contain radioactive elements.
The two-step process ensures the zirconolite phase—which acts as the host for radionuclides—is distributed evenly throughout the material.
Ensuring Structural Integrity
A uniform, fine-grained structure is mechanically superior to a coarse, irregular one.
By controlling the thermal path, engineers prevent the formation of large crystals that could introduce stress points or reduce the chemical durability of the matrix.
Understanding Process Sensitivities
The Risk of Thermal Overlap
A major trade-off in this process is the narrow margin for error regarding temperature control.
If the temperature in the first stage is too high, it may inadvertently trigger crystal growth alongside nucleation. This results in fewer, larger crystals, which defeats the purpose of the two-step approach.
Time-Temperature Dependence
The duration of the hold times is just as critical as the temperature settings.
Holding the first stage for too long produces no additional benefit once saturation nucleation is reached, while extending the second stage can lead to Ostwald ripening, where larger crystals consume smaller ones, degrading the microstructural uniformity.
Optimizing the Thermal Path for Your Goals
To achieve the best results in preparing zirconolite-based matrices, consider your specific containment requirements:
- If your primary focus is maximum radionuclide loading: Prioritize the first stage (nucleation) to ensure the highest possible density of zirconolite sites available to incorporate waste elements.
- If your primary focus is mechanical durability: Strictly control the maximum temperature and duration of the second stage to prevent grain coarsening, which ensures a tougher, more fracture-resistant matrix.
Precise thermal management is the difference between a standard glass material and a high-performance nuclear waste barrier.
Summary Table:
| Stage | Purpose | Temperature Target | Impact on Microstructure |
|---|---|---|---|
| Stage One | Nucleation | Slightly above $T_g$ | Creates high-density microscopic crystal nuclei |
| Stage Two | Crystal Growth | High Growth Zone | Facilitates uniform, fine-grained zirconolite crystals |
| Result | Immobilization | Controlled Thermal Path | Ensures structural integrity and high radionuclide loading |
Elevate Your Material Stability with KINTEK
Achieving the precise thermal path required for high-performance glass-ceramics demands uncompromising temperature control. Backed by expert R&D and manufacturing, KINTEK offers high-precision Muffle, Tube, and Vacuum systems—all fully customizable to meet the rigorous demands of nuclear waste immobilization and advanced material science.
Ready to optimize your two-step heat treatment? Contact our experts today to find the perfect furnace solution for your lab.
Visual Guide
References
- S. V. Yudintsev, V. I. Malkovsky. Thermal Effects and Glass Crystallization in Composite Matrices for Immobilization of the Rare-Earth Element–Minor Actinide Fraction of High-Level Radioactive Waste. DOI: 10.3390/jcs8020070
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 600T Vacuum Induction Hot Press Vacuum Heat Treat and Sintering Furnace
People Also Ask
- What is the temperature of a sintering furnace? From 1100°C to 2200°C+ for Your Material
- What is the primary function of glass matrices in HLW vitrification? Achieve Safe Radioactive Waste Immobilization
- What is the purpose of using controlled anaerobic environments for peat carbonization? Unlock High-Energy Industrial Fuel
- Why is a laboratory vacuum evaporation system essential for the preparation of electrodes in high-performance solar cells?
- Why is a constant temperature drying oven used at 120°C for 16 hours for NiCuCe catalysts? Optimize Site Dispersion
- What are the advantages of HTL reactors for algae? Optimize Biomass Conversion Without Pre-Drying
- What is the purpose of using high-purity argon gas for NAB alloys? Ensure Superior Nickel-Aluminum Bronze Integrity
- Why is ALD equipment used for rear passivation of silicon solar cells? Optimize Your PERC and TOPCon Efficiency



















