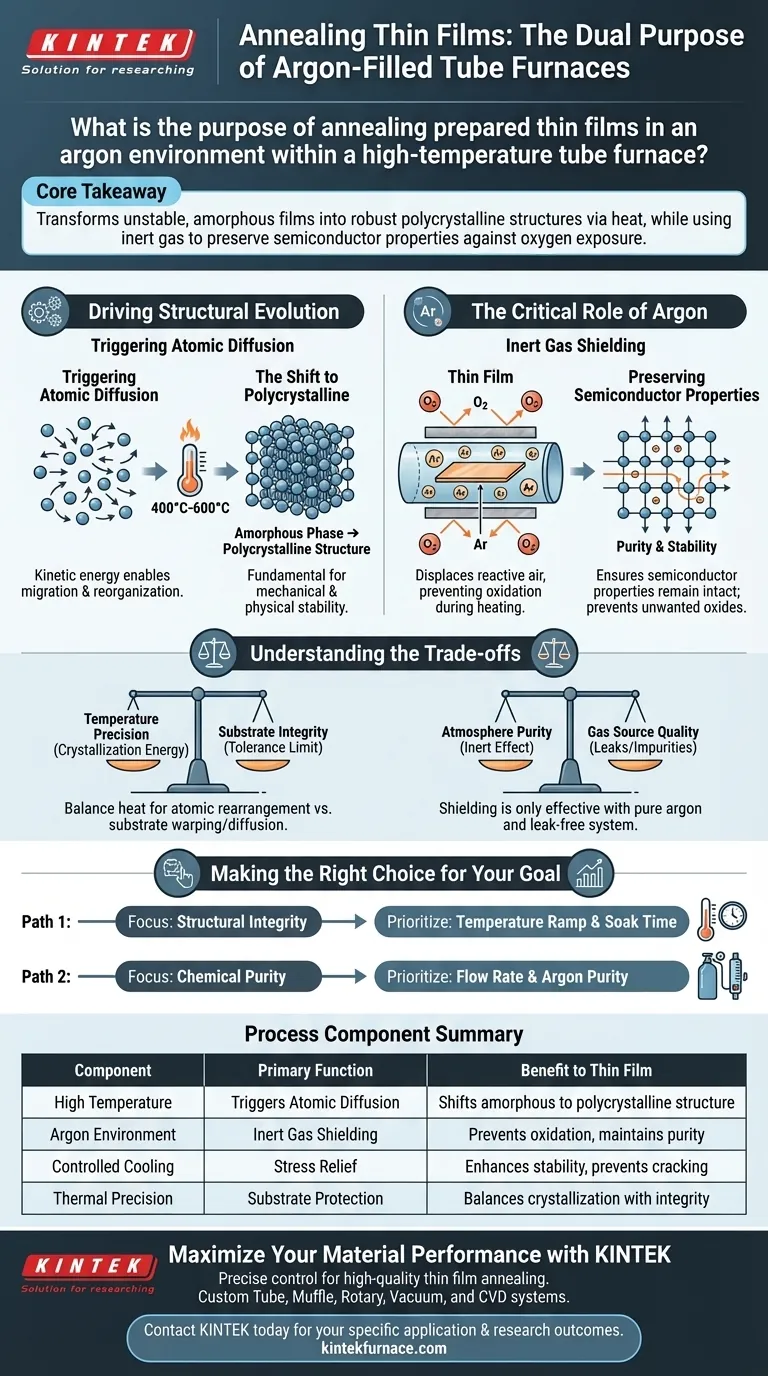
Annealing thin films in an argon-filled tube furnace serves a dual critical function: structural optimization and chemical protection. This process subjects the film to a controlled thermal environment—typically between 400°C and 600°C—to drive necessary atomic rearrangement, while the argon atmosphere acts as an inert shield to prevent the material from degrading via oxidation.
Core Takeaway: The process is designed to transform unstable, amorphous films into robust polycrystalline structures via heat, while simultaneously using inert gas to preserve the material's semiconductor properties against oxygen exposure.

Driving Structural Evolution
Triggering Atomic Diffusion
Freshly prepared thin films often exist in a disordered or amorphous state.
By raising the temperature within the furnace, you provide the kinetic energy required for atomic diffusion. This allows atoms within the film to migrate and reorganize into a lower-energy configuration.
The Shift to Polycrystalline
The primary goal of this rearrangement is crystallization.
The heat treatment drives the transformation from an amorphous phase to a ordered polycrystalline structure. This structural organization is fundamental to establishing the mechanical and physical stability of the film.
The Critical Role of Argon
Inert Gas Shielding
High temperatures dramatically accelerate chemical reactions, particularly oxidation.
If annealed in air, many thin films would react with oxygen, destroying their intended chemical composition. Argon functions as an inert shielding gas, displacing reactive air to create a safe environment for the heating process.
Preserving Semiconductor Properties
For semiconductor films, purity is paramount.
By preventing oxidation, argon ensures the stability of the semiconductor properties. It allows the physical structure to improve (crystallize) without allowing the chemical structure to degrade or convert into an unwanted oxide.
Understanding the Trade-offs
Temperature Precision vs. Substrate Integrity
While higher temperatures generally promote better crystallization, there is an upper limit.
You must balance the heat required for atomic rearrangement against the tolerance of your substrate. Excessive heat can cause substrate warping or unwanted interlayer diffusion, effectively ruining the device.
Atmosphere Purity
The "shielding" effect is only as good as the purity of your gas source.
Using argon is ineffective if the tube furnace has leaks or if the gas supply contains impurities. Even trace amounts of oxygen at 600°C can compromise the film's conductive or optical performance.
Making the Right Choice for Your Goal
When configuring your annealing process, prioritize your parameters based on your specific material requirements:
- If your primary focus is Structural Integrity: Prioritize the temperature ramp and soak time to ensure complete transformation from amorphous to polycrystalline.
- If your primary focus is Chemical Purity: Prioritize the flow rate and purity of the argon gas to ensure zero oxidation occurs during the thermal cycle.
Effective annealing balances thermal energy for growth with chemical isolation for protection.
Summary Table:
| Process Component | Primary Function | Benefit to Thin Film |
|---|---|---|
| High Temperature | Triggers Atomic Diffusion | Shifts amorphous state to stable polycrystalline structure |
| Argon Environment | Inert Gas Shielding | Prevents oxidation and maintains semiconductor purity |
| Controlled Cooling | Stress Relief | Enhances mechanical stability and prevents film cracking |
| Thermal Precision | Substrate Protection | Balances crystallization energy with substrate integrity |
Maximize Your Material Performance with KINTEK
Precise control over temperature and atmosphere is non-negotiable for high-quality thin film annealing. Backed by expert R&D and manufacturing, KINTEK offers high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique laboratory requirements.
Whether you are scaling semiconductor research or perfecting advanced coatings, our furnaces provide the thermal stability and inert gas integrity your projects demand. Contact KINTEK today to discuss your specific application and discover how our specialized heating solutions can elevate your research outcomes.
Visual Guide
References
- Joun Ali Faraz, Kamran Ahmad. Photoelectrochemical Water Splitting by SnO2/CuO Thin Film Heterostructure-Based Photocatalysts for Hydrogen Generation. DOI: 10.3390/nano15221748
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- How is a high-temperature resistance furnace used to evaluate TiN coatings? Ensure Reliability for Aerospace Parts
- What components make up the atmosphere control system of the box type annealing atmosphere furnace? Discover Key Parts for Precise Heat Treatment
- What types of gases are commonly used in atmosphere furnaces and why? Optimize Your Heat Treatment Process
- What are the common applications of box-type atmosphere furnaces? Essential for High-Temperature Controlled Environments
- What is the function of a tube atmosphere furnace? Precision Mn1/CeO2 Catalyst Reduction & Fabrication
- How does a continuous controlled atmosphere furnace operate? Unlock High-Volume Precision in Material Processing
- Why is it necessary to use an atmosphere furnace with argon gas? Ensure Precise Alloy Austenitization & Protection
- How does the versatility of a controlled atmosphere furnace benefit material processing? Unlock Precise Material Engineering



















