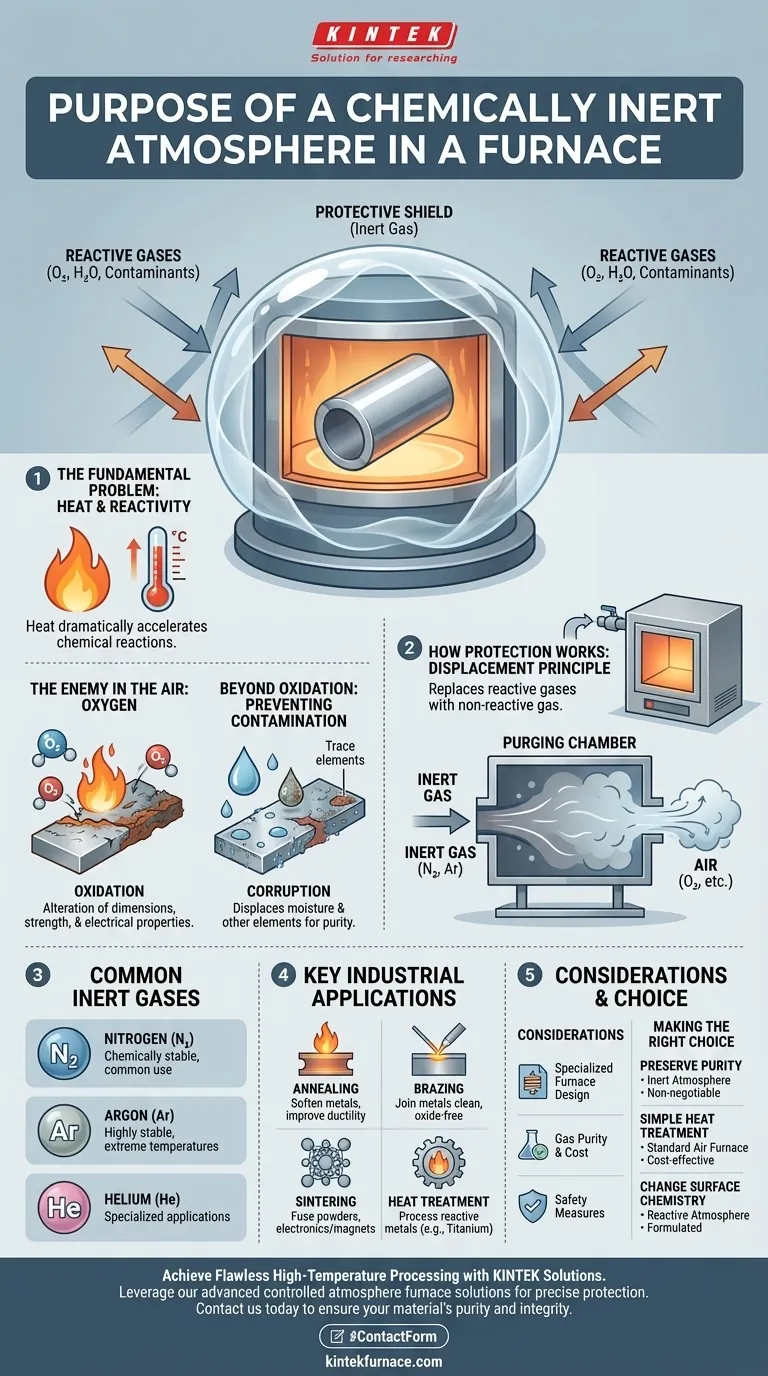
At its core, the purpose of a chemically inert atmosphere in a furnace is to create a protective shield around a material during high-temperature processing. This shield displaces reactive gases like oxygen from the air, preventing unwanted chemical reactions such as oxidation and contamination that would otherwise degrade the material's quality and properties.
The fundamental role of an inert atmosphere is to ensure that the only change occurring to a material in a furnace is the one you intend from the heat itself. It isolates the workpiece from its environment, preserving its chemical purity and structural integrity at elevated temperatures.

The Fundamental Problem: Heat and Reactivity
To understand the need for an inert atmosphere, we must first recognize that heat dramatically accelerates chemical reactions. A material that is stable at room temperature can become highly reactive when heated in a furnace.
The Role of High Temperatures
Heat provides the energy required for atoms to break existing bonds and form new ones. This makes high-temperature processes like annealing, sintering, or brazing inherently vulnerable to unwanted chemical changes.
The Enemy in the Air: Oxygen
The most common adversary in this environment is oxygen. When metals are heated in the presence of oxygen, they rapidly oxidize, forming a layer of scale or rust on the surface. This oxidation can alter the material's dimensions, strength, and electrical properties.
Beyond Oxidation: Preventing Contamination
Air also contains moisture and other trace elements that can contaminate a sensitive workpiece. An inert atmosphere displaces these elements, ensuring the final product remains pure and meets precise specifications.
How an Inert Atmosphere Provides Protection
A controlled atmosphere furnace doesn't remove reactive gases; it replaces them entirely with a gas that will not participate in any chemical reactions.
The Principle of Displacement
Before the heating process begins, the furnace chamber is purged with an inert gas. This gas, typically heavier than air, fills the chamber and physically pushes out the oxygen, moisture, and other contaminants, leaving only a non-reactive environment surrounding the workpiece.
Common Inert Gases: Nitrogen and Argon
Nitrogen (N2) and Argon (Ar) are the most frequently used gases for this purpose. They are chosen because they are chemically stable and do not readily react with other elements, even at extreme temperatures. Helium is also used in some specialized applications.
Key Industrial Applications
This technique is critical in processes where material integrity is paramount. Common applications include:
- Annealing: Softening metals to improve ductility without surface oxidation.
- Brazing: Joining metals with a filler material in a clean, oxide-free environment.
- Sintering: Fusing powdered materials, such as in semiconductor or magnetic component manufacturing.
- Heat Treatment: Processing highly reactive metals like titanium alloys that are extremely sensitive to oxygen.
Understanding the Trade-offs and Considerations
While essential for many applications, operating with an inert atmosphere introduces complexity and requires specialized equipment.
Specialized Furnace Design
These processes cannot be performed in a standard oven. They require a sealed controlled atmosphere furnace designed to contain the gas, prevent leaks, and allow for proper purging.
Gas Purity and Consumption
The effectiveness of the shield depends on the purity of the inert gas. Any contamination in the gas supply can compromise the process. This also represents an ongoing operational cost.
Critical Safety Measures
Controlled atmosphere furnaces are sophisticated systems. Because some processes may involve flammable gases in addition to inert ones, they demand strict safety protocols, including gas monitoring systems, explosion-proof devices, and operation by trained personnel.
Making the Right Choice for Your Goal
Selecting the right furnace atmosphere depends entirely on the desired outcome for your material.
- If your primary focus is preserving absolute material purity and preventing any surface oxidation: An inert atmosphere is non-negotiable.
- If your primary focus is simple heat treatment where slight surface discoloration or scaling is acceptable: A standard furnace that operates in ambient air may be sufficient and more cost-effective.
- If your primary focus is actively changing the material's surface chemistry (e.g., carburizing): You would need a specifically formulated reactive atmosphere, not an inert one.
Ultimately, using an inert atmosphere is a deliberate choice to control every variable and ensure the material exiting the furnace is exactly what you designed.
Summary Table:
| Purpose | Mechanism | Common Gases | Key Applications |
|---|---|---|---|
| Prevent Oxidation | Displaces oxygen from air to stop scale/rust formation | Nitrogen (N₂), Argon (Ar) | Annealing, Heat Treatment |
| Prevent Contamination | Shields material from moisture & trace elements in air | Helium (specialized uses) | Brazing, Sintering |
| Preserve Material Integrity | Creates a non-reactive environment for pure thermal processing | Semiconductor Manufacturing |
Achieve Flawless High-Temperature Processing with KINTEK Solutions
Does your application require precise protection from oxidation and contamination? Leveraging exceptional R&D and in-house manufacturing, KINTEK provides diverse laboratories with advanced controlled atmosphere furnace solutions. Our product line, including Vacuum & Atmosphere Furnaces, Tube Furnaces, and CVD/PECVD Systems, is complemented by our strong deep customization capability to precisely meet your unique experimental requirements.
Contact us today to discuss how our expertise can ensure your material's purity and integrity. Let's build your perfect thermal processing solution together.
Visual Guide
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does nitrogen atmosphere heat treatment improve surface strengthening? Enhance Durability and Performance
- What is the main purpose of heat treatment? Transform Metal Properties for Superior Performance
- What does inert mean in furnace atmospheres? Protect materials from oxidation with inert gases.
- What is the use of nitrogen in furnace? Prevent Oxidation for Superior Heat Treatment
- How does a chemically inert atmosphere function in a furnace? Prevent Oxidation and Ensure Material Purity



















