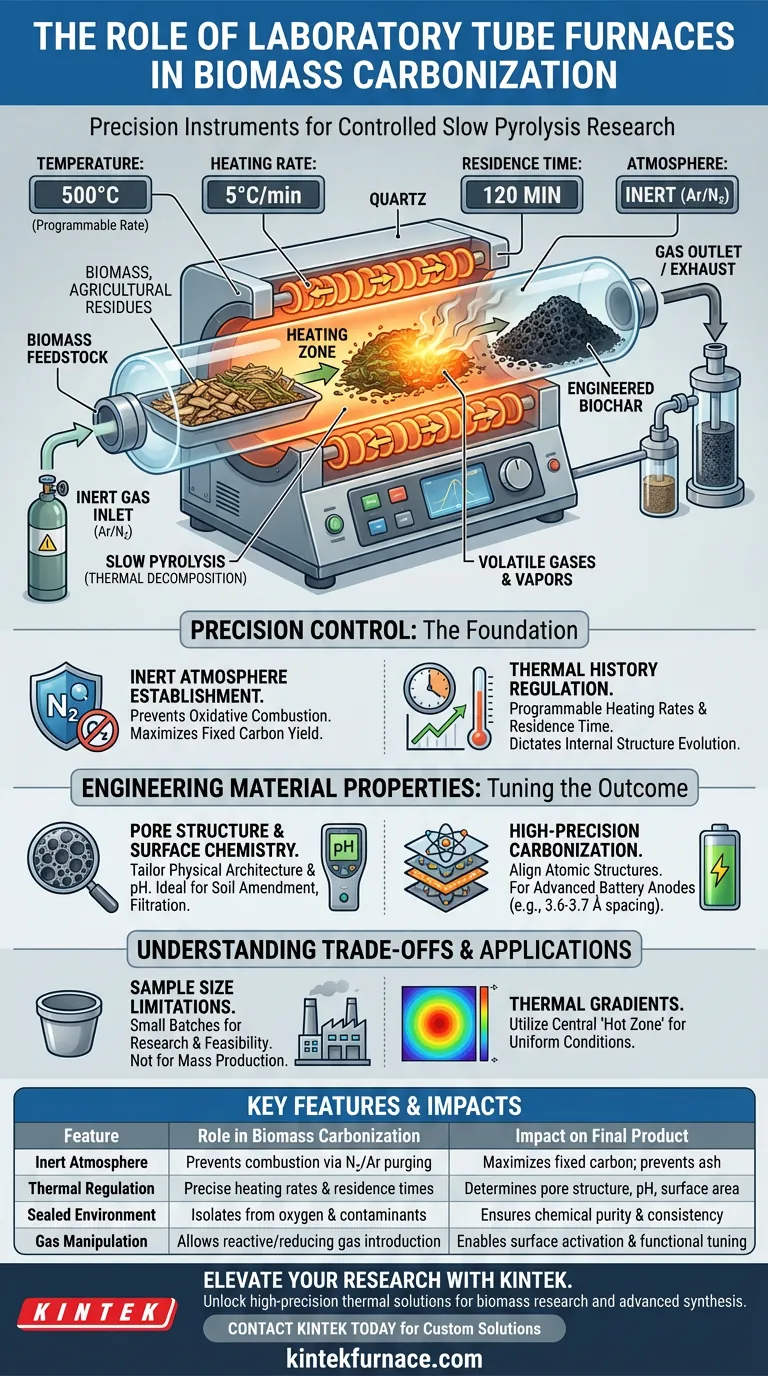
The primary function of a laboratory tube furnace in biomass carbonization is to facilitate research into slow pyrolysis within a strictly controlled environment. By isolating the biomass in a sealed heating chamber, typically under an inert or reactive atmosphere, the furnace allows you to convert organic feedstock into biochar without the risk of oxidative combustion. This equipment enables the precise manipulation of heating rates and residence times, which are the critical variables that determine the final properties of the carbonized material.
By decoupling the thermal process from ambient oxygen, a tube furnace acts as a precision instrument for material engineering. It allows researchers to isolate specific variables—such as temperature ramps and gas environments—to determine exactly how they influence the pore structure, pH, and fixed carbon content of the resulting biochar.

The Role of Precision Control
Establishing the Inert Atmosphere
The fundamental requirement for carbonization is the absence of oxygen. A tube furnace excels here by utilizing a sealed quartz or alumina tube that can be evacuated or purged with inert gases like argon or nitrogen.
This setup ensures that the biomass undergoes pyrolysis—thermal decomposition—rather than combustion. By preventing the raw materials from burning to ash, the furnace maximizes the yield of fixed carbon and preserves the carbon skeleton.
Regulating Thermal History
The transformation of biomass is highly sensitive to how heat is applied. Tube furnaces utilize electrical resistance heating to provide uniform thermal radiation and conduction to the sample.
This allows for programmable heating rates (e.g., 5 °C/min). Precise control over the ramp-up speed and the duration of isothermal holding (residence time) directly dictates the evolution of the material's internal structure.
Engineering Material Properties
Tuning Pore Structure and Surface Chemistry
The specific "recipe" of heat and time applied in a tube furnace governs the physical architecture of the biochar. Researchers use these furnaces to study how different thermal treatments expand or collapse the material's pores.
Additionally, the process influences the chemical surface of the char. By adjusting conditions, you can manipulate the pH value and the removal of oxygen-containing functional groups, tailoring the biochar for specific applications like soil amendment or filtration.
Achieving High-Precision Carbonization
For advanced applications, such as battery anodes, the transition from raw cellulose to hard carbon requires exacting standards. A tube furnace provides the stable thermal environment needed to align atomic structures.
High-precision temperature control ensures consistent interlayer spacing (often around 3.6-3.7 Å) and proper graphitization degrees. This level of consistency is virtually impossible to achieve in less controlled, open-air heating methods.
Understanding the Trade-offs
Sample Size Limitations
While tube furnaces offer unmatched control, they are inherently limited by volume. The cylindrical heating chamber restricts the sample size to small batches, making them ideal for research and feasibility studies but unsuitable for mass production.
Thermal Gradients
Although designed for uniformity, temperature gradients can exist near the ends of the tube where insulation is thinner. It is critical to ensure the sample is positioned centrally within the "hot zone" to guarantee that the entire biomass batch experiences the exact same thermal conditions.
Making the Right Choice for Your Research
To get the most out of a laboratory tube furnace, align your operational parameters with your specific research objectives:
- If your primary focus is Biochar Characterization: Prioritize slow heating rates and long residence times to meticulously study changes in pore structure and pH values.
- If your primary focus is Advanced Material Synthesis: Utilize high-vacuum or strictly controlled inert gas flows (like Argon) to ensure high-purity carbonization and precise atomic arrangement.
- If your primary focus is Surface Activation: Consider introducing reactive reducing gases (such as hydrogen mixtures) to strip oxygen functional groups without collapsing the pore network.
The laboratory tube furnace is the bridge between raw organic mass and engineered carbon materials, transforming variables into tangible, reproducible results.
Summary Table:
| Feature | Role in Biomass Carbonization | Impact on Final Product |
|---|---|---|
| Inert Atmosphere | Prevents oxidative combustion via nitrogen/argon purging | Maximizes fixed carbon yield; prevents ash formation |
| Thermal Regulation | Precise control of heating rates and residence times | Determines pore structure, surface area, and pH |
| Sealed Environment | Isolates biomass from ambient oxygen and contaminants | Ensures chemical purity and consistent material properties |
| Gas Manipulation | Allows introduction of reactive or reducing gases | Enables surface activation and functional group tuning |
Elevate Your Carbonization Research with KINTEK
Unlock the full potential of your material engineering with KINTEK’s high-precision thermal solutions. Backed by expert R&D and world-class manufacturing, we provide a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems—all fully customizable to meet the exacting standards of biomass research and advanced synthesis.
Whether you are refining biochar pore structures or developing advanced battery anodes, our laboratory furnaces deliver the uniform heating and atmospheric control necessary for reproducible results. Contact KINTEK today to discuss your unique needs and let our experts help you design the perfect furnace for your laboratory.
Visual Guide
References
- Waheed A. Rasaq, Andrzej Białowiec. Navigating Pyrolysis Implementation—A Tutorial Review on Consideration Factors and Thermochemical Operating Methods for Biomass Conversion. DOI: 10.3390/ma17030725
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What is the role of a high-temperature Tube Furnace in copper alloy homogenization? Enhance Material Ductility
- How does a drop tube furnace compare to a horizontal tube furnace? Choose the Right Furnace for Your Process
- What functions does a tube atmosphere furnace perform for high-entropy alloy catalysts? Essential Reduction & Control
- Why use a tube furnace with atmosphere control for NiFe LDH to NiFe alloy conversion? Achieve Precise Metal Reduction
- What are the advantages of multi-zone tube furnaces? Achieve Superior Thermal Control for Advanced Materials Processing
- How does a high-temperature tube furnace facilitate HfOC/SiOC pyrolysis? Mastering Polymer-to-Ceramic Transition
- What are the technical advantages of using SPS vs. tube furnaces for SiC? Achieve Superior SiC Properties
- How does a tube furnace facilitate the carbonization of ZIFs while preventing oxidation? Expert Insights



















