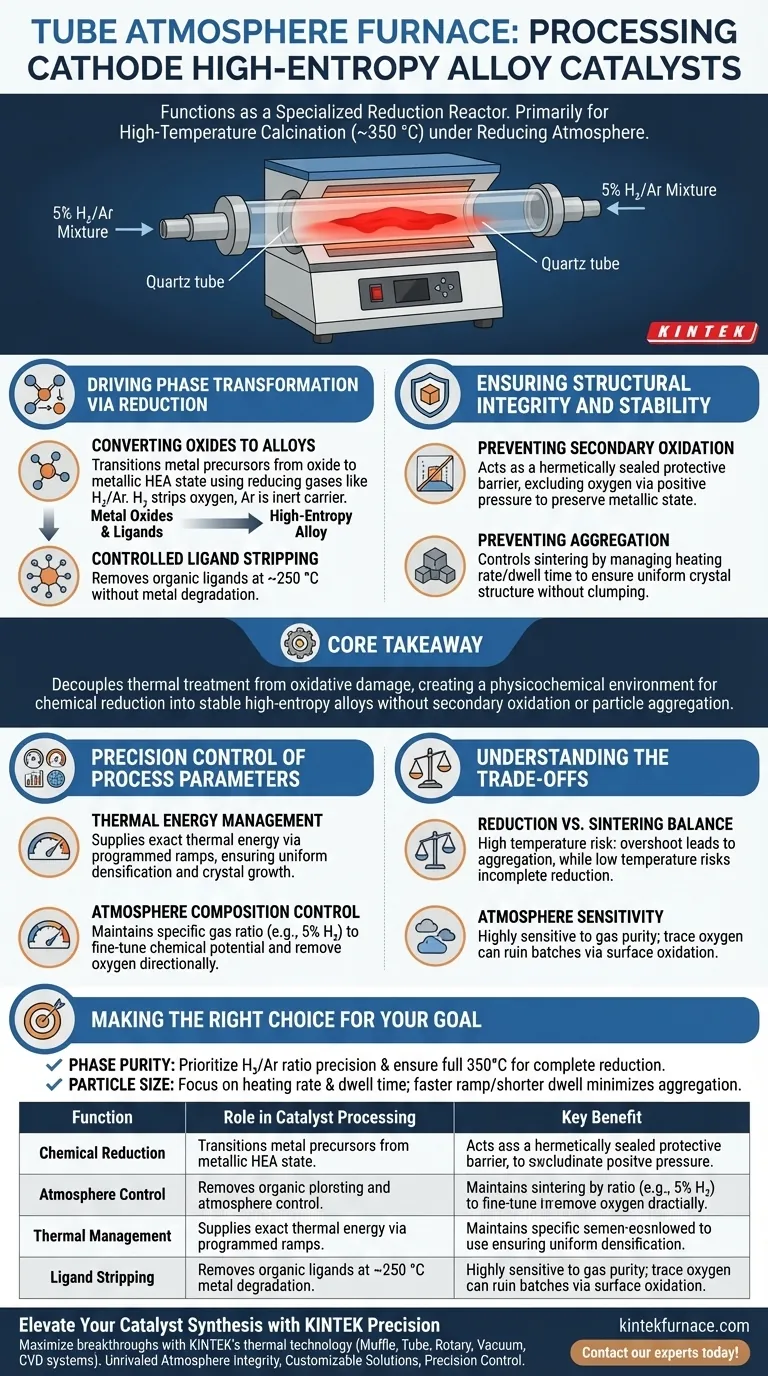
A tube atmosphere furnace functions as a specialized reduction reactor during the processing of cathode high-entropy alloy catalysts. It is primarily used to perform high-temperature calcination—typically around 350 °C—following initial treatments like microwave processing. By maintaining a specific reducing atmosphere (such as a 5% Hydrogen/Argon mixture), the furnace supplies the necessary thermal energy to convert metal oxides into a high-entropy alloy state while strictly preventing oxidation.
Core Takeaway The tube atmosphere furnace is essential for decoupling thermal treatment from oxidative damage. It creates a physicochemical environment that drives the chemical reduction of metal precursors into stable high-entropy alloys, ensuring the catalyst achieves the precise microstructure required for performance without suffering from secondary oxidation or particle aggregation.

Driving Phase Transformation via Reduction
Converting Oxides to Alloys
The primary function of the furnace is to facilitate a chemical phase change. It transitions metal precursors from an oxide state into a metallic, high-entropy alloy state.
This is achieved not just through heat, but through the introduction of reducing gases, such as a hydrogen/argon mixture. The hydrogen acts as the reducing agent, stripping oxygen from the metal oxides, while the argon provides an inert carrier medium.
Controlled Ligand Stripping
Before the alloy forms, the furnace helps remove organic ligands attached to the metal precursors.
Operating at controlled temperatures (often beginning around 250 °C), the reducing environment allows for "ligand stripping." This removes unwanted organic components without causing the metal atoms to react unpredictably or degrade.
Ensuring Structural Integrity and Stability
Preventing Secondary Oxidation
High-entropy alloys are highly reactive during formation. Without protection, the high temperatures required for synthesis would immediately cause the metals to re-oxidize in open air.
The furnace acts as a hermetically sealed protective barrier. By maintaining positive pressure with the gas mixture, it ensures that oxygen is excluded from the chamber, preserving the metallic nature of the newly formed alloy.
Preventing Aggregation
A critical challenge in catalyst synthesis is keeping active particles small and dispersed.
The furnace controls the "sintering" effect. By precisely managing the heating rate and dwell time, it ensures the metal atoms form the desired crystal structure (the high-entropy state) without clumping together into large, less effective masses.
Precision Control of Process Parameters
Thermal Energy Management
The furnace supplies the exact thermal energy required to overcome the activation energy barriers for alloy formation.
This is not a blast of heat, but a programmed ramp. The ability to control the temperature gradient ensures that densification and crystal growth occur uniformly, rather than chaotically.
Atmosphere Composition Control
The specific ratio of gases (e.g., 5% H2 vs. 95% Ar) is maintained to fine-tune the chemical potential inside the tube.
This allows for the directional removal of oxygen. It enables the adjustment of the chemical properties of the active sites without damaging the underlying support structure or pores of the catalyst material.
Understanding the Trade-offs
The Balance of Reduction vs. Sintering
While the furnace enables reduction, high temperatures always carry the risk of "overshoot."
If the dwell time is too long or the temperature slightly too high, the nanoparticles may aggregate, reducing the active surface area. Conversely, if the temperature is too low, the reduction of the high-entropy alloy may be incomplete, leaving behind inactive oxides.
Atmosphere Sensitivity
The process is highly sensitive to the gas mixture purity.
Even trace amounts of oxygen due to a leak or impure gas supply can ruin the batch by causing surface oxidation. The "protective barrier" function of the furnace is only as good as the integrity of the seals and the quality of the input gas.
Making the Right Choice for Your Goal
To optimize the processing of cathode high-entropy alloy catalysts, align your furnace parameters with your specific structural goals:
- If your primary focus is Phase Purity: Prioritize the precision of the Hydrogen/Argon ratio and ensure the temperature reaches the full 350 °C threshold to guarantee complete reduction of oxides to the alloy state.
- If your primary focus is Particle Size (Surface Area): Focus on the heating rate and dwell time; a faster ramp with a shorter dwell can often achieve reduction while minimizing the window for particle aggregation.
Success depends on using the furnace not just as a heater, but as a precision instrument to manage the competition between alloy formation and particle growth.
Summary Table:
| Function | Role in Catalyst Processing | Key Benefit |
|---|---|---|
| Chemical Reduction | Converts metal oxides to alloy state using H₂/Ar gas. | Ensures phase purity and metallic state. |
| Atmosphere Control | Provides a hermetically sealed, oxygen-free environment. | Prevents secondary oxidation and contamination. |
| Thermal Management | Precise programmed ramping and dwell times. | Minimizes particle aggregation (sintering). |
| Ligand Stripping | Removes organic precursors at controlled temperatures. | Prepares clean metal sites for alloy formation. |
Elevate Your Catalyst Synthesis with KINTEK Precision
Maximize your research breakthroughs with KINTEK’s industry-leading thermal technology. Backed by expert R&D and world-class manufacturing, we offer Muffle, Tube, Rotary, Vacuum, and CVD systems designed for the rigorous demands of high-entropy alloy development.
Why choose KINTEK?
- Unrivaled Atmosphere Integrity: Achieve the ultra-pure reducing environments necessary for HEA phase stability.
- Customizable Solutions: Our systems are tailored to your unique high-temp lab requirements.
- Precision Control: Master the balance between reduction and sintering with advanced thermal programming.
Ready to optimize your material performance? Contact our experts today to find your perfect furnace solution!
Visual Guide
References
- Chiung-Wen Chang, Shih‐Yuan Lu. High performance anion exchange membrane water electrolysis driven by atomic scale synergy of non-precious high entropy catalysts. DOI: 10.20517/energymater.2025.05
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the role of a laboratory tube furnace in teaching and training? Enhance Student Learning with Hands-On Thermal Experiments
- What role do furnace chamber working conditions play in selecting a vertical tube furnace? Ensure Optimal Performance and Process Success
- What physical conditions does a high-temperature tube furnace provide? Optimize Lignin Carbonization Success
- What core functions does an argon atmosphere tube furnace perform? Optimize Al-PTFE FGM Sintering
- What is the primary purpose of using a high-temperature tube furnace? Master nZVI@BC Synthesis with Precision
- What role does a high-purity quartz tube furnace play in graphene growth? Achieve Conformal Optical Resonator Coating
- What role does a three-zone tube furnace play in converting 6FDA-TFDB-x precursors? Precision CMS Membrane Carbonization
- How did the tube furnace originate and where is it commonly used today? Discover Its Evolution and Modern Applications



















