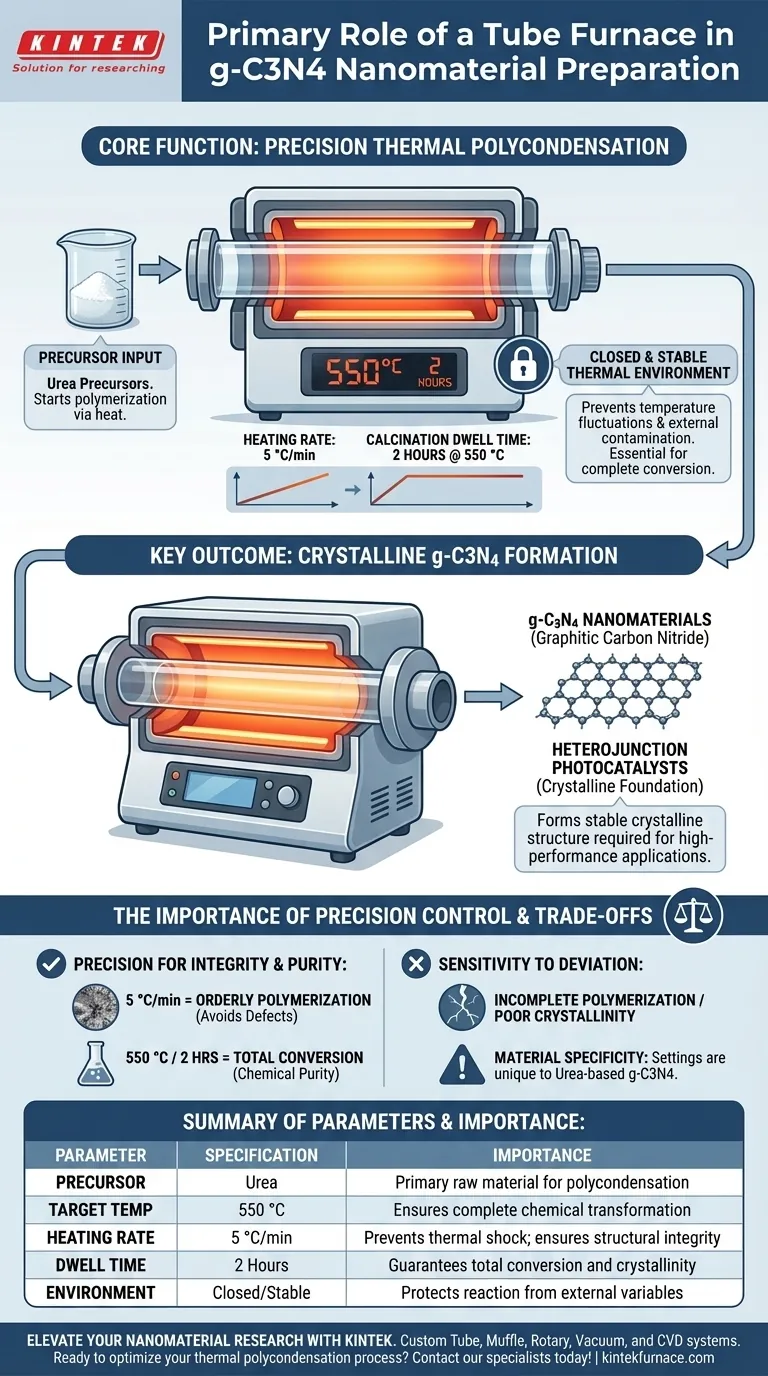
The primary role of a Tube Furnace in the preparation of g-C3N4 nanomaterials is to generate a closed, strictly controlled thermal environment essential for the thermal polycondensation of urea. It acts as the reaction vessel that drives the chemical transformation of precursor materials into a stable crystalline structure.
The Tube Furnace serves as a precision instrument rather than a simple heater. By enforcing specific heating rates and dwell times, it ensures the complete calcination of urea, establishing the crystalline foundation required for high-performance heterojunction photocatalysts.

The Mechanics of Thermal Polycondensation
Creating a Closed Reaction Environment
To synthesize graphitic carbon nitride (g-C3N4), the reaction area must be isolated from uncontrolled external variables. The Tube Furnace provides a closed and stable high-temperature environment. This stability is critical for preventing temperature fluctuations that could disrupt the formation of the nanomaterial's structure.
Facilitating Precursor Conversion
The furnace is responsible for the thermal polycondensation of the starting material. In this specific application, urea precursors are subjected to heat to initiate polymerization. The furnace ensures that the thermal energy is applied uniformly, driving the chemical changes necessary to convert raw urea into the desired g-C3N4 nanomaterials.
The Importance of Precision Control
Regulating the Heating Rate
Success in nanomaterial synthesis often depends on how fast the temperature rises. The Tube Furnace allows for a precisely controlled heating rate of 5 °C/min. This gradual ramp-up prevents thermal shock and allows the polymerization process to occur in an orderly fashion.
Maintaining Target Temperature and Duration
Reaching the correct temperature is only half the battle; maintaining it is equally important. The furnace must achieve a target temperature of 550 °C and hold this constant calcination state for 2 hours. This duration ensures the complete conversion of the precursors, leaving no unreacted urea behind.
Defining Crystalline Structure
The ultimate goal of these strict parameters is the formation of specific crystalline structures. By adhering to the 550 °C / 2-hour protocol, the furnace facilitates the creation of a material quality suitable for constructing heterojunction photocatalysts.
Understanding the Trade-offs
Sensitivity to Parameter Deviation
The primary advantage of the Tube Furnace—precision—is also its main operational constraint. The synthesis of g-C3N4 is highly sensitive to the defined parameters. A deviation from the 5 °C/min heating rate or the 550 °C setpoint can lead to incomplete polymerization or poor crystallinity.
Material Specificity
While Tube Furnaces are versatile tools capable of synthesizing various nanomaterials like nanoparticles and nanowires (via CVD methods), this specific process relies on thermal polycondensation. Users must understand that the settings used for urea-based g-C3N4 are specific to that precursor and may not translate directly to other substrate materials or synthesis methods.
Making the Right Choice for Your Goal
To maximize the effectiveness of your Tube Furnace in g-C3N4 synthesis, consider your specific research objectives:
- If your primary focus is Structural Integrity: Adhere strictly to the 5 °C/min ramp rate to ensure the crystalline lattice forms correctly without defects caused by rapid heating.
- If your primary focus is Chemical Purity: Ensure the 2-hour calcination at 550 °C is fully completed to guarantee total conversion of the urea precursor.
Precision in your thermal profile is the single most important factor in determining the quality of your final photocatalytic material.
Summary Table:
| Parameter | Specification for g-C3N4 | Importance |
|---|---|---|
| Precursor | Urea | Primary raw material for polycondensation |
| Target Temperature | 550 °C | Ensures complete chemical transformation |
| Heating Rate | 5 °C/min | Prevents thermal shock; ensures structural integrity |
| Dwell Time | 2 Hours | Guarantees total conversion and crystallinity |
| Environment | Closed/Stable | Protects reaction from external variables |
Elevate Your Nanomaterial Research with KINTEK
Precision is the difference between a failed reaction and a high-performance photocatalyst. At KINTEK, we understand that g-C3N4 synthesis requires absolute control over thermal profiles. Backed by expert R&D and world-class manufacturing, we offer high-performance Tube, Muffle, Rotary, Vacuum, and CVD systems—all fully customizable to meet your unique laboratory requirements.
Ready to optimize your thermal polycondensation process? Contact our specialists today to find your perfect furnace solution!
Visual Guide
References
- Rahil Azhar, W.I. Nawawi. Effect of Different Preparation Approaches on Pt-Modified TiO2/g-C3N4 for Effective Photocatalytic Degradation of RR4 Dye Under Visible Light. DOI: 10.24191/srj.v22i2.31241
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What features are included in a standard tube furnace system? Essential Components for Precise Thermal Processing
- What are the benefits of high yield and product concentration in a tube furnace? Boost Efficiency and Purity in Chemical Processes
- What role does a laboratory tube furnace play in the carbonization process of moxa floss? Expert Guide to Biomass Synthesis
- How do tube furnaces and muffle furnaces differ in design and application? Choose the Right Furnace for Your Lab
- What are the benefits of integrating multiple heating zones in a tube furnace? Unlock Precise Thermal Control
- How does a split tube furnace compare to non-split tube furnaces? Choose the Right Furnace for Your Lab
- What are the technical advantages of using a high-temperature tube furnace? Precision Thermal Oxidation Explained
- What core functions does a tube high-temperature furnace perform? Mastering In-situ Carbothermal Reduction



















