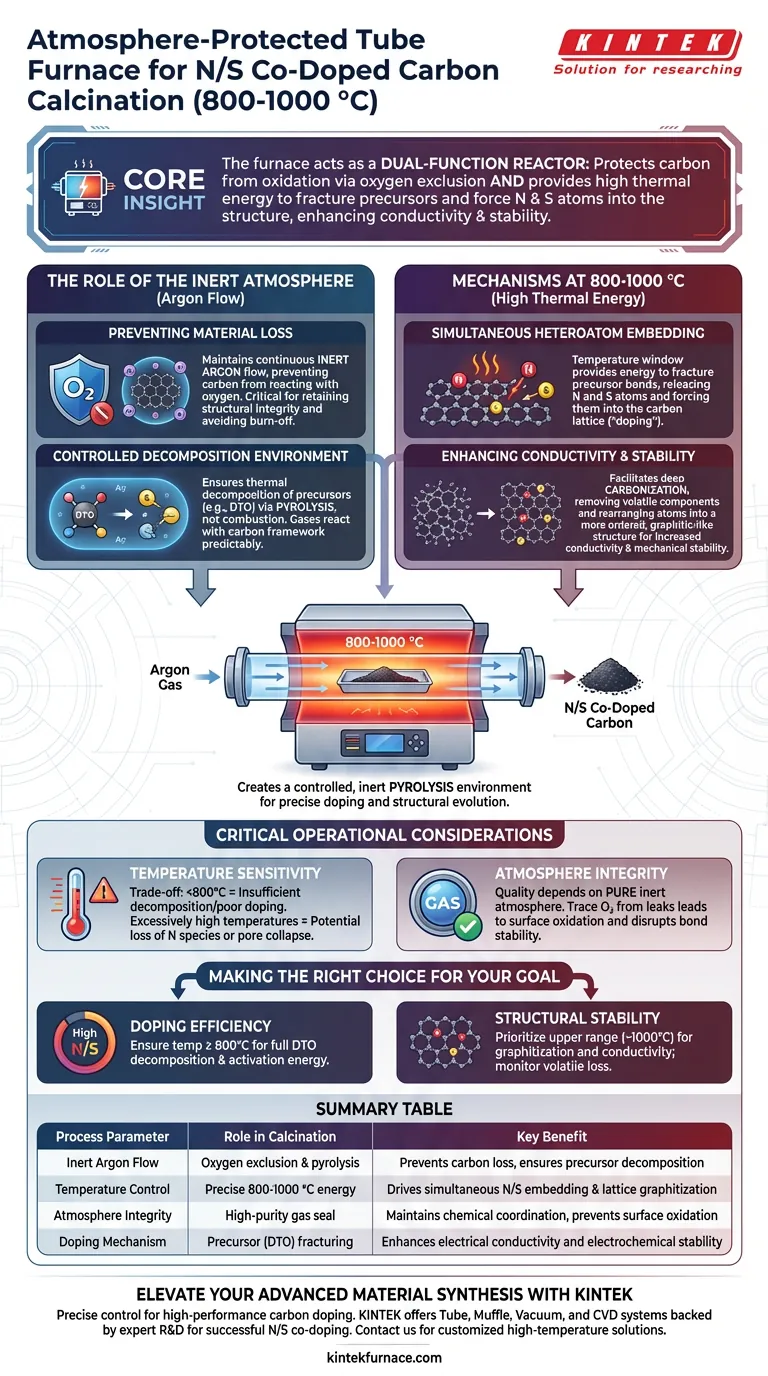
The primary purpose is to create a controlled, inert pyrolysis environment that drives precise chemical doping and structural evolution. At temperatures between 800 °C and 1000 °C, an atmosphere-protected tube furnace utilizes an argon atmosphere to facilitate the decomposition of specific precursors like dithiooxamide (DTO). This environment is essential for simultaneously embedding nitrogen and sulfur atoms into the carbon lattice while preventing material oxidation.
Core Insight: The atmosphere-protected tube furnace acts as a dual-function reactor: it protects the carbon framework from burning away via oxygen exclusion while providing the high thermal energy required to fracture precursor molecules and force nitrogen and sulfur atoms into the carbon structure, thereby enhancing conductivity and stability.
The Role of the Inert Atmosphere
Preventing Material Loss
The most immediate function of the tube furnace is oxygen exclusion. By maintaining a continuous flow of inert argon gas, the system prevents the carbon material from reacting with oxygen.
Without this protection, the high processing temperatures would cause the carbon precursor to oxidize and burn off rather than graphitize. This protection is critical for retaining the structural integrity of the hard carbon materials during heat treatment.
Controlled Decomposition Environment
The inert atmosphere provides a stable background for chemical reactions. It ensures that the thermal decomposition of precursors, such as dithiooxamide (DTO), occurs via pyrolysis rather than combustion.
This specific environment allows DTO to decompose into sulfur-containing gases in a predictable manner. These gases are then available to react directly with the carbon framework without interference from atmospheric contaminants.
Mechanisms at 800-1000 °C
Simultaneous Heteroatom Embedding
The 800-1000 °C temperature window is energetic enough to drive the simultaneous embedding of nitrogen and sulfur atoms.
The thermal energy fractures the chemical bonds of the precursor materials. This releases nitrogen and sulfur atoms and forces them into the defects and lattice structure of the carbon, effectively "doping" the material.
Enhancing Conductivity and Stability
Beyond simple doping, this temperature range facilitates deep carbonization.
The heat treatment removes volatile non-carbon components and rearranges the carbon atoms into a more ordered, graphitic-like structure. This structural evolution significantly increases the material's electrical conductivity and mechanical stability, which are vital for electrochemical applications.
Critical Operational Considerations
Temperature Sensitivity
Operating within the specific 800-1000 °C range is a careful trade-off.
If the temperature is too low (e.g., below 800 °C), the thermal energy may be insufficient to fully decompose the DTO or drive the sulfur atoms into the carbon lattice, resulting in poor doping efficiency. Conversely, excessively high temperatures could lead to the loss of nitrogen species or the collapse of the pore structure.
Atmosphere Integrity
The quality of the final product is entirely dependent on the purity of the inert atmosphere.
Even trace amounts of oxygen due to leaks or impure argon can lead to surface oxidation. This disrupts the formation of the desired coordination environment and can degrade the stability of the nitrogen and sulfur bonds within the framework.
Making the Right Choice for Your Goal
To maximize the efficacy of your synthesis, align your furnace parameters with your specific material requirements:
- If your primary focus is doping efficiency: Ensure your temperature reaches at least 800 °C to fully decompose DTO and provide the activation energy needed for sulfur and nitrogen to bond chemically with the carbon.
- If your primary focus is structural stability: Prioritize the upper end of the temperature range (approaching 1000 °C) to maximize graphitization and electrical conductivity, but monitor for potential loss of volatile dopants.
Success relies on balancing the thermal energy required for doping against the need to preserve the active sites within the carbon matrix.
Summary Table:
| Process Parameter | Role in Calcination (800-1000 °C) | Key Benefit |
|---|---|---|
| Inert Argon Flow | Oxygen exclusion & pyrolysis environment | Prevents carbon loss and ensures precursor decomposition |
| Temperature Control | Precise 800-1000 °C thermal energy | Drives simultaneous N/S embedding and lattice graphitization |
| Atmosphere Integrity | High-purity gas seal | Maintains chemical coordination and prevents surface oxidation |
| Doping Mechanism | Precursor (DTO) fracturing | Enhances electrical conductivity and electrochemical stability |
Elevate Your Advanced Material Synthesis with KINTEK
Precise control over atmosphere and temperature is non-negotiable for high-performance carbon doping. KINTEK provides industry-leading Tube, Muffle, Vacuum, and CVD systems designed to meet the rigorous demands of your lab. Backed by expert R&D and manufacturing, our furnaces offer the stability needed for successful N/S co-doping and structural evolution.
Need a customized high-temperature solution? Contact us today to discover how KINTEK's precision-engineered equipment can enhance your research and manufacturing outcomes.
Visual Guide
References
- Jiahui Li, Shaobo Tu. Pseudocapacitive Heteroatom‐Doped Carbon Cathode for Aluminum‐Ion Batteries with Ultrahigh Reversible Stability. DOI: 10.1002/eem2.12733
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What temperature range and applications is this tube furnace suitable for? Ideal for 500°C to 1800°C thermal processes
- What is a Tube Furnace? Master Precision Heating for Sensitive Materials
- Why are controlled atmosphere and vacuum operations important for tube furnaces? Protect Materials and Enable Precision Reactions
- What conditions does a tube furnace provide for aluminum ash-based ceramsite roasting? Master Precision Sintering
- Why is environment control in a tube furnace critical for NASICON? Optimize Ionic Conductivity and Density
- Why is a high-precision tube furnace required for PtCln/Fe-N-C catalysts? Ensure Sub-Nanometer Precision
- What common processes are enabled by tube furnaces? Unlock Precise Thermal Processing for Your Lab
- How does high-temperature heating tape function in conjunction with a tube furnace? Optimize Methanol Cracking.



















