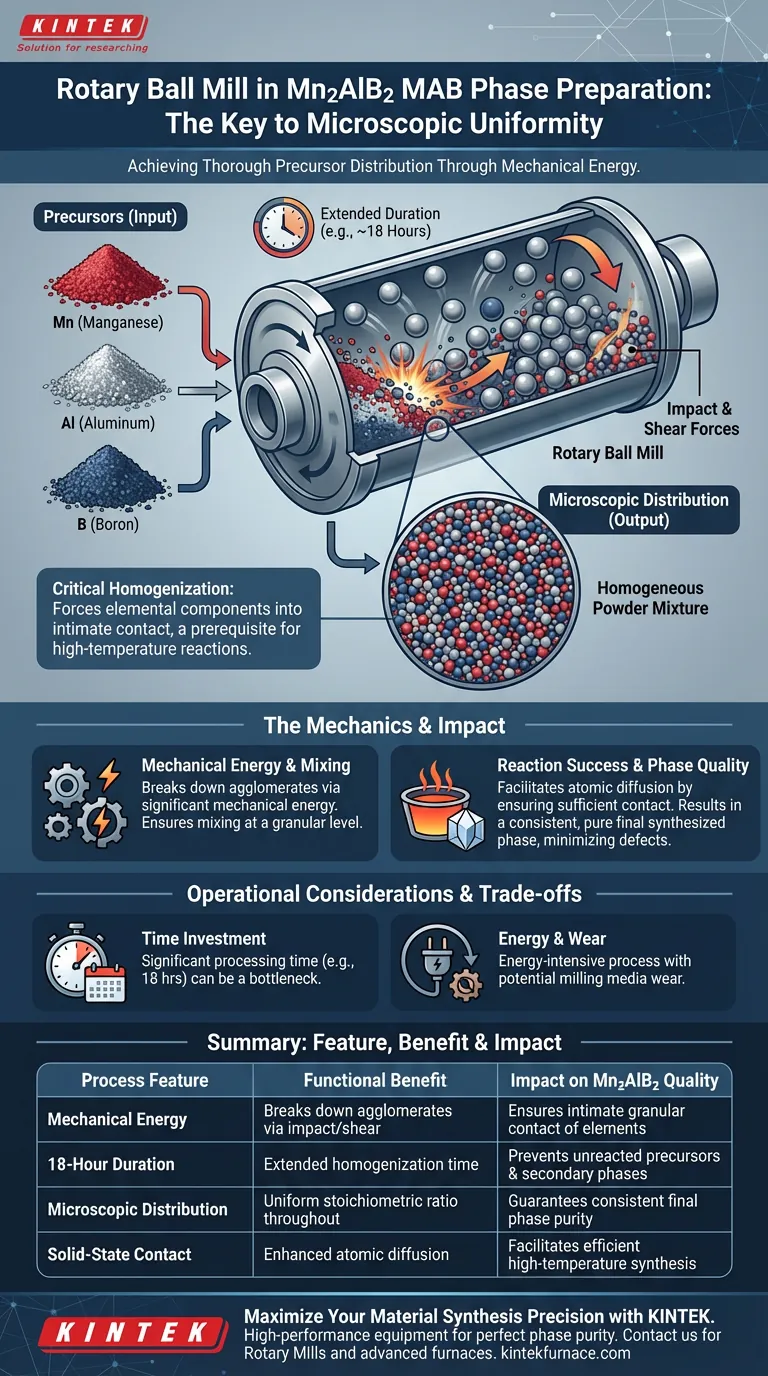
The primary purpose of using a rotary ball mill in the preparation of Mn2AlB2 MAB phase powder is to achieve a thorough, microscopic distribution of the precursor components. By applying mechanical energy over an extended duration, typically around 18 hours, the mill ensures that manganese, aluminum, and boron powders are mixed far more uniformly than simple blending could achieve.
Rotary ball milling acts as a critical homogenization step that forces elemental components into intimate contact. This microscopic uniformity is the prerequisite for successful high-temperature reactions, ensuring the final synthesized phase is consistent and pure.
The Mechanics of Homogenization
Utilizing Mechanical Energy
The rotary ball mill does not merely stir the ingredients; it imparts significant mechanical energy into the powder mixture.
As the mill rotates, the impact and shear forces break down particle agglomerates. This forces the disparate elements—manganese, aluminum, and boron—to mix at a granular level.
Achieving Microscopic Distribution
The goal of this process is uniform microscopic distribution.
In solid-state synthesis, having the correct average composition is not enough; the elements must be mixed perfectly at the microscopic scale. The ball milling process ensures that every distinct region of the powder mixture contains the correct stoichiometric ratio of elements.
Impact on Reaction Success
Facilitating Contact
For solid-state reactions to occur, reactant particles must be in physical contact to allow for atomic diffusion.
The extended milling duration ensures that all elements are in sufficient contact with one another. This proximity is vital for the subsequent high-temperature heat treatment to proceed efficiently.
Ensuring Final Phase Quality
The ultimate output of this rigorous mixing is the homogeneity of the final synthesized phase.
Without the intimate mixing provided by the ball mill, the final product would likely contain unreacted precursors or unwanted secondary phases. The mechanical processing minimizes these defects, leading to a purer Mn2AlB2 MAB phase.
Operational Considerations and Trade-offs
Time Investment
A key consideration in this process is the duration required for effectiveness.
The reference specifically notes an extended processing time, such as 18 hours. This makes the milling step a significant time investment in the overall production cycle, acting as a potential bottleneck for rapid throughput.
Energy and Wear
The reliance on continuous mechanical energy implies a trade-off in terms of energy consumption.
While necessary for quality, the process is energy-intensive compared to simpler mixing methods. Additionally, the mechanical action can introduce wear on the milling media, requiring careful monitoring to prevent contamination (though contamination is not explicitly detailed in the reference, it is a standard implication of mechanical milling).
Optimizing Your Synthesis Strategy
To ensure the best results when synthesizing Mn2AlB2, align your processing parameters with your quality requirements.
- If your primary focus is High Phase Purity: Adhere strictly to extended milling times (e.g., 18 hours) to guarantee the microscopic element contact required for a homogeneous final product.
- If your primary focus is Process Consistency: Standardize the mechanical energy input and duration across all batches to ensure reproducible stoichiometric distribution.
Thorough mechanical milling is the foundational step that dictates the success of the entire synthesis process.
Summary Table:
| Process Feature | Functional Benefit | Impact on Mn2AlB2 Quality |
|---|---|---|
| Mechanical Energy | Breaks down agglomerates via impact/shear | Ensures intimate granular contact of elements |
| 18-Hour Duration | Extended homogenization time | Prevents unreacted precursors & secondary phases |
| Microscopic Distribution | Uniform stoichiometric ratio throughout | Guarantees consistent final phase purity |
| Solid-State Contact | Enhanced atomic diffusion | Facilitates efficient high-temperature synthesis |
Maximize Your Material Synthesis Precision with KINTEK
High-performance materials like Mn2AlB2 MAB phases demand rigorous preparation and thermal processing. KINTEK provides the cutting-edge equipment necessary to achieve perfect phase purity and consistency. Backed by expert R&D and manufacturing, we offer high-precision Rotary Mills, Muffle, Tube, Vacuum, and CVD systems, all customizable to your specific research or industrial needs.
Don't let inconsistent mixing or thermal gradients compromise your results. Contact KINTEK today to discover how our advanced lab furnaces and milling solutions can optimize your production workflow.
Visual Guide
References
- Fatma Nur Tuzluca Yesilbag, Ahmad Hüseyin. The effect of Aluminum (Al) ratio on the synthesis of the laminated Mn2AlB2 MAB Phase. DOI: 10.18185/erzifbed.1514470
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Kiln for Pyrolysis Plant Heating
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Chairside Dental Porcelain Zirconia Sintering Furnace with Transformer for Ceramic Restorations
People Also Ask
- What materials are commonly used for furnace tubes to withstand high heat? Choose the Best for Your Lab
- What types of trays are compatible with MoSi2 heating elements? Ensure Optimal Performance and Longevity
- What are the primary functions of the vacuum pump system and inert gases? Achieve High-Purity Atomization
- Why use a fusion furnace and platinum crucibles for XRF analysis of magnesium slag? Ensure Accurate Results
- What role does a heat-resistant steel retort play in sintering? Mastering Isolation and Pressure for High-Purity Results
- Why are corundum crucibles selected for high-temperature annealing of LiScO2:Cr3+? Protect Purity and Performance
- Why is high-purity graphite paper typically lined on the inner walls of the mold before loading Ti-6Al-4V alloy powder?
- Why must fuel injectors used in high-temperature furnace systems incorporate a cooling function? Prevent Coking Today



















