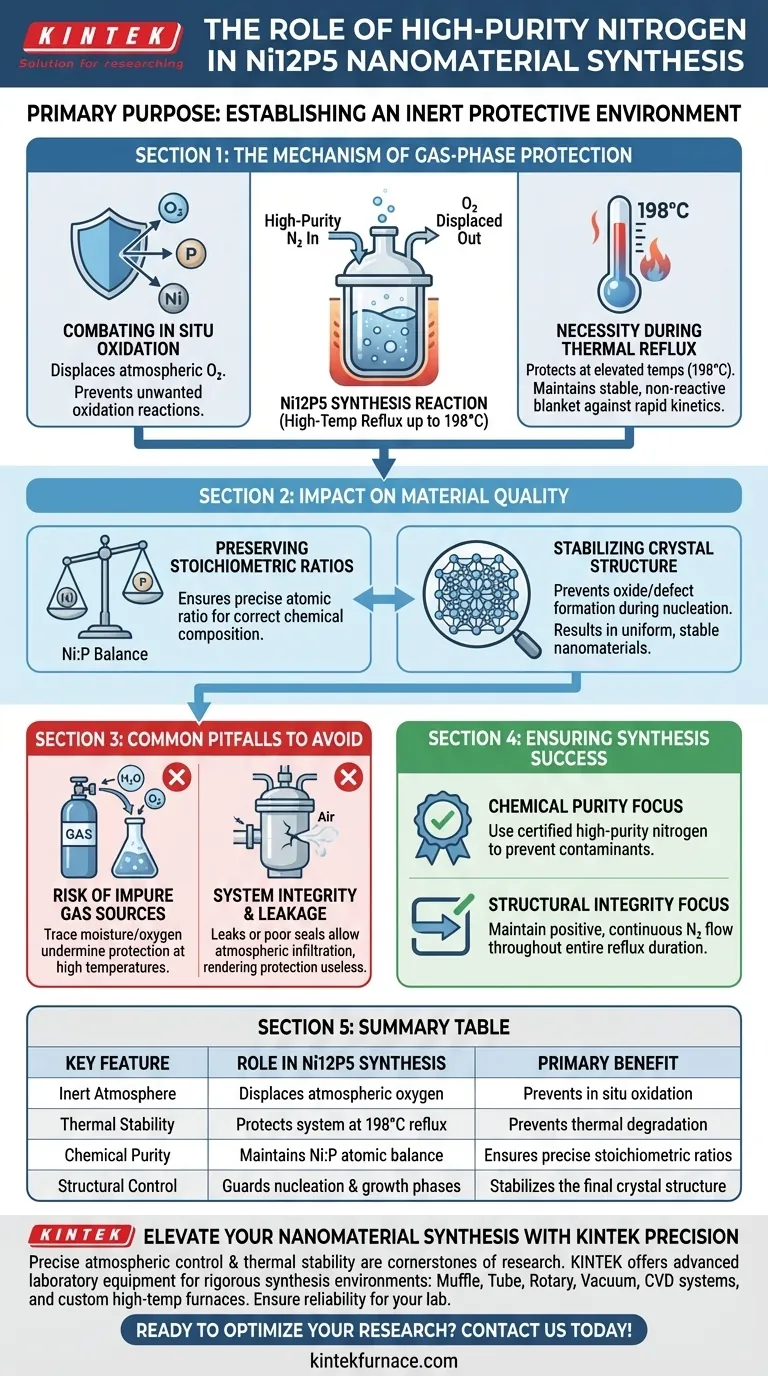
The primary purpose of introducing high-purity nitrogen gas is to establish an inert protective environment. During the chemical synthesis of Ni12P5 nanomaterials, this gas shield prevents the reaction system from undergoing in situ oxidation. This protection is particularly critical during high-temperature reflux processes, which can reach up to 198°C.
By effectively displacing atmospheric oxygen, high-purity nitrogen ensures the resulting nickel phosphides maintain their precise stoichiometric ratio and crystal structure stability throughout the heating phase.

The Mechanism of Gas-Phase Protection
Combating In Situ Oxidation
The chemical synthesis of metal phosphides like Ni12P5 is highly sensitive to the surrounding atmosphere. If left exposed, the reactive components will interact with oxygen rather than forming the intended compound.
High-purity nitrogen acts as a physical barrier. It displaces air within the reaction vessel, preventing in situ oxidation from compromising the chemical pathway.
The Necessity During Thermal Reflux
This synthesis relies on reflux processes that generate significant heat, specifically reaching temperatures around 198°C.
At these elevated temperatures, reaction kinetics accelerate, and the materials become exponentially more susceptible to oxidative damage. Nitrogen maintains a stable, non-reactive blanket over the mixture, ensuring the high thermal energy drives the synthesis forward rather than degradation.
Impact on Material Quality
Preserving Stoichiometric Ratios
The defining characteristic of Ni12P5 is the specific atomic ratio of nickel to phosphorus.
Oxidation introduces a variable that disrupts this balance, potentially creating impurities or alternative phases of nickel phosphide. The inert nitrogen environment ensures the reactants combine exactly as intended to achieve the correct stoichiometry.
Stabilizing Crystal Structure
The functional properties of nanomaterials are dictated by their internal arrangement.
By preventing the formation of oxides or defects during the nucleation and growth phases, nitrogen gas ensures the crystal structure stability of the final product. This leads to a more uniform and predictable nanomaterial.
Common Pitfalls to Avoid
The Risk of Impure Gas sources
While the goal is an inert environment, the quality of the nitrogen source matters.
Using nitrogen that is not "high-purity" can introduce trace amounts of moisture or oxygen into the system. Even minor impurities can act as contaminants at 198°C, undermining the protective effect and altering the material properties.
System Integrity and Leakage
Introducing gas is only effective if the reaction vessel remains sealed against the outside atmosphere.
A common oversight is failing to maintain positive pressure or having leaks in the reflux setup. If the nitrogen flow is interrupted or the seal is imperfect, atmospheric oxygen will infiltrate the system, rendering the protective measure useless.
Ensuring Synthesis Success
To achieve high-quality Ni12P5 nanomaterials, apply the following principles to your synthesis protocol:
- If your primary focus is Chemical Purity: Ensure the nitrogen source is certified high-purity to prevent trace contaminants from altering the specific stoichiometric ratio.
- If your primary focus is Structural Integrity: Maintain a continuous, positive flow of nitrogen throughout the entire 198°C reflux duration to fully protect the crystal structure stability.
Strict adherence to atmosphere control is the baseline requirement for reproducible, high-performance nanomaterial synthesis.
Summary Table:
| Key Feature | Role in Ni12P5 Synthesis | Primary Benefit |
|---|---|---|
| Inert Atmosphere | Displaces atmospheric oxygen | Prevents in situ oxidation |
| Thermal Stability | Protects system at 198°C reflux | Prevents thermal degradation |
| Chemical Purity | Maintains Ni:P atomic balance | Ensures precise stoichiometric ratios |
| Structural Control | Guards nucleation & growth phases | Stabilizes the final crystal structure |
Elevate Your Nanomaterial Synthesis with KINTEK Precision
Precise atmospheric control and thermal stability are the cornerstones of high-performance nanomaterial research. KINTEK provides the advanced laboratory equipment necessary to maintain rigorous synthesis environments.
Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with other lab high-temp furnaces—all fully customizable to meet your unique chemical synthesis needs. Whether you are combating oxidation or perfecting stoichiometric ratios, KINTEK ensures your lab has the reliability it deserves.
Ready to optimize your research results? Contact us today to find the perfect furnace solution for your lab!
Visual Guide
References
- Omkar V. Vani, Anil M. Palve. Solar‐Powered Remediation of Carcinogenic Chromium(VI) and Methylene Blue Using Ferromagnetic Ni<sub>12</sub>P<sub>5</sub> and Porous Ni<sub>12</sub>P<sub>5</sub>‐rGO Nanostructures. DOI: 10.1002/metm.70010
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1200℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
- 1700℃ Controlled Inert Nitrogen Atmosphere Furnace
- Mesh Belt Controlled Atmosphere Furnace Inert Nitrogen Atmosphere Furnace
- Controlled Inert Nitrogen Hydrogen Atmosphere Furnace
People Also Ask
- How does a high-temperature pyrolysis furnace convert EFB fibers to biochar? Master Precise Thermal Carbonization
- What challenges are associated with inert atmosphere furnaces? Overcome High Costs and Complexity
- What is the purpose of an atmosphere furnace? Control Gas Environments for Superior Material Processing
- What is the primary function of a tube atmosphere furnace? Mastering Ti3AlC2 MAX Phase Synthesis
- What is the primary structural difference between a muffle furnace and an atmosphere furnace? Control Gas for Better Results
- What is a box-type atmosphere furnace? Master Controlled Heat for Material Processing
- Why is it necessary to use an atmosphere furnace for MOF melt-quenching? Protect Fragile Materials from Decomposition
- How does the heating and cooling performance of box type atmosphere furnaces benefit production? Boost Throughput and Quality



















