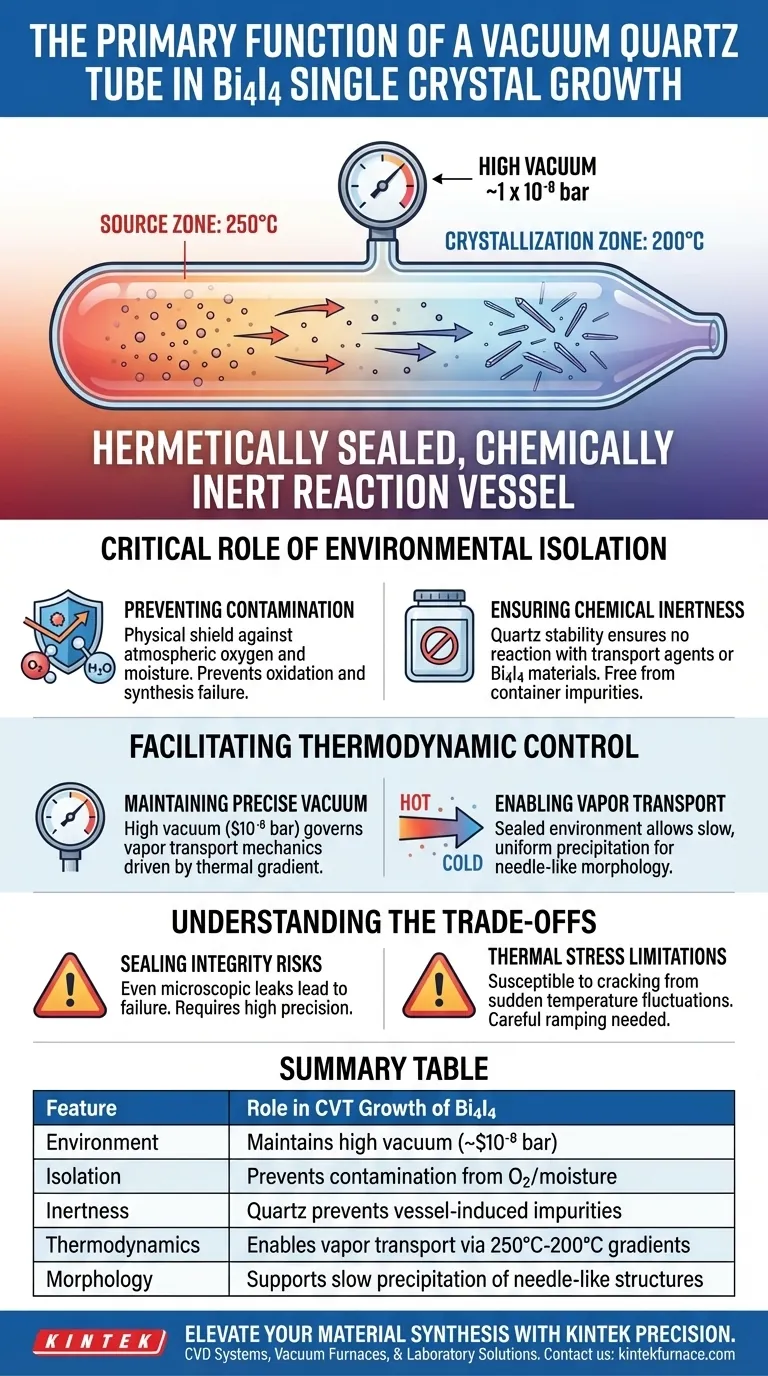
The vacuum quartz tube serves as a hermetically sealed, chemically inert reaction vessel. In the chemical vapor transport (CVT) method for growing Bi4I4 single crystals, its primary function is to maintain a high-vacuum environment of approximately $1 \times 10^{-8}$ bar. This isolation is critical to prevent raw materials from reacting with atmospheric oxygen or moisture while ensuring the precise pressure conditions necessary for high-purity crystal formation.
By creating an isolated, high-vacuum environment, the quartz tube serves as the foundational barrier against contamination, facilitating the precise pressure conditions required to grow high-purity, needle-like Bi4I4 crystals.

The Critical Role of Environmental Isolation
Preventing Chemical Contamination
The synthesis of Bi4I4 occurs at elevated temperatures where raw materials become highly reactive. The vacuum quartz tube acts as a physical shield.
It explicitly prevents the precursors from oxidizing or interacting with atmospheric moisture. Without this barrier, external contaminants would degrade the stoichiometry and destroy the crystal quality.
Ensuring Chemical Inertness
Beyond simply isolating the reaction, the vessel itself must remain passive. Quartz is utilized because of its high chemical stability.
It does not react with the transport agents or the Bi4I4 source materials. This ensures that the resulting single crystals are free from container-induced impurities.
Facilitating Thermodynamic Control
Maintaining Precise Vacuum Pressure
The CVT process relies on specific pressure dynamics to function correctly. The tube allows for the maintenance of a high vacuum, specifically around $1 \times 10^{-8}$ bar.
This low-pressure environment is necessary to govern the vapor transport mechanics. It ensures the process is driven by the thermal gradient rather than interference from background gases.
Enabling Vapor Transport
Inside the sealed tube, gaseous substances migrate from the high-temperature source zone ($250^\circ\text{C}$) to the low-temperature crystallization zone ($200^\circ\text{C}$).
The tube contains this volatile ecosystem. It allows the Bi4I4 to precipitate slowly and uniformly, resulting in the desired needle-like morphology.
Understanding the Trade-offs
Sealing Integrity Risks
The effectiveness of the entire growth process relies on a perfect seal. Even a microscopic leak in the quartz tube defeats the purpose of the vacuum immediately.
Compromised integrity leads to oxidation and total synthesis failure. The sealing process requires high technical precision to withstand the duration of the growth cycle.
Thermal Stress Limitations
While quartz is heat-resistant, it is not immune to thermal shock. The tube must withstand the stress of the temperature gradient without cracking or devitrifying.
Sudden fluctuations in the furnace temperature can compromise the structural integrity of the tube. This physical limitation requires careful ramping of temperature protocols.
Optimizing the Growth Environment
To ensure successful Bi4I4 synthesis, the preparation of the reaction vessel is as important as the thermal profile itself.
- If your primary focus is chemical purity: Ensure the tube is evacuated to a strict vacuum of $1 \times 10^{-8}$ bar to completely eliminate atmospheric oxygen and moisture.
- If your primary focus is crystal morphology: Verify that the tube is sealed securely to maintain constant pressure, allowing for the slow, uniform precipitation of needle-like structures.
Meticulous preparation of the vacuum quartz tube is the invisible variable that dictates the difference between a failed experiment and high-quality topological materials.
Summary Table:
| Feature | Role in CVT Growth of Bi4I4 |
|---|---|
| Environment | Maintains high vacuum (~1 x 10⁻⁸ bar) for transport |
| Isolation | Prevents contamination from atmospheric O2 and moisture |
| Inertness | Quartz material prevents vessel-induced impurities |
| Thermodynamics | Enables vapor transport via precise 250°C to 200°C gradients |
| Morphology | Supports slow precipitation of needle-like crystal structures |
Elevate Your Material Synthesis with KINTEK Precision
Achieving the perfect topological material requires more than just a recipe; it demands a controlled environment. Backed by expert R&D and manufacturing, KINTEK offers high-performance CVD systems, vacuum furnaces, and customizable laboratory solutions designed to maintain the rigorous vacuum and thermal profiles your research demands.
Whether you are growing Bi4I4 single crystals or developing next-generation semiconductors, our systems are tailored for your unique needs. Contact KINTEK today to discuss how our advanced furnace technology can ensure the purity and morphology of your high-temp materials.
Visual Guide
References
- Dong Chen, Claudia Felser. Observation of Surface 2D Electron Gas in Highly Bulk‐Insulating Bi<sub>4</sub>I<sub>4</sub>. DOI: 10.1002/andp.202500136
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Multi Heating Zones CVD Tube Furnace Machine for Chemical Vapor Deposition Equipment
People Also Ask
- How is a laboratory tube furnace utilized in the thermal shock reduction process to produce RGO?
- Why is a high-precision tube furnace required during Fe-Mn catalyst synthesis? Control Morphology and CNF Quality
- How does a three-zone tube furnace facilitate the synthesis of germanium nanowires? Achieve High-Quality SVG Results
- How are vacuum tube furnaces utilized in the metallurgical industry? Enhance Metal Purity and Performance
- What is the primary function of a high-temperature tube furnace in HELMA synthesis? Achieve 1500°C Precision
- How does a high-temperature tube furnace facilitate the ammonolysis process? Master TiNx Nanoparticle Synthesis
- Why is the vertical orientation of a drop tube furnace significant? Unlock Superior Process Control and Efficiency
- What is the function of the high-purity quartz tube in CVT for ZrTe5? Ensure High Purity and Vacuum Integrity



















