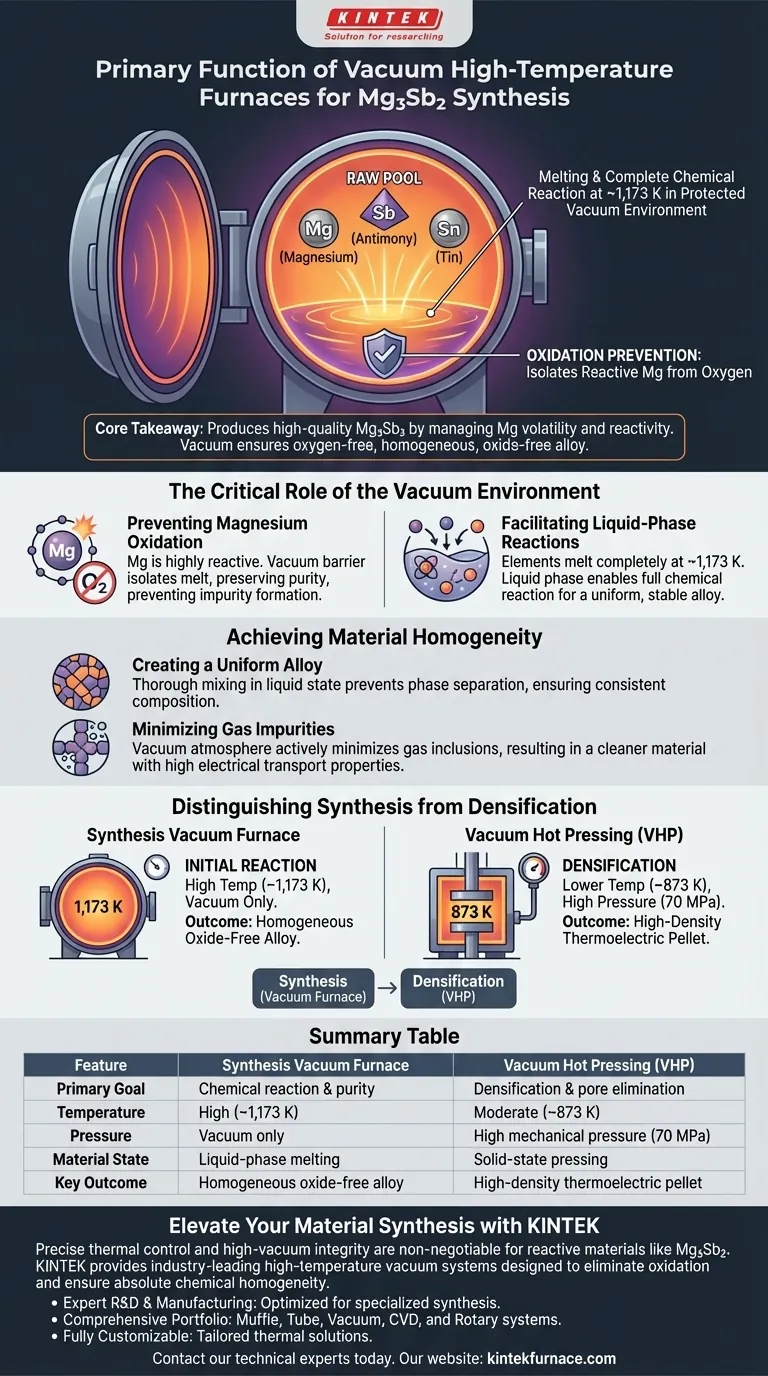
The primary function of a vacuum high-temperature furnace during the synthesis of Mg3Sb2 is to enable the melting and complete chemical reaction of raw elements—specifically Magnesium (Mg), Antimony (Sb), and Tin (Sn)—in a protected environment. By operating at temperatures around 1,173 K within a vacuum, the furnace facilitates a liquid-phase reaction while strictly preventing the oxidation of the highly reactive magnesium.
Core Takeaway Producing high-quality Mg3Sb2 requires managing the volatility and reactivity of Magnesium. The vacuum furnace solves this by providing an oxygen-free thermal environment, allowing the raw elements to mix into a homogeneous, oxide-free alloy before further processing.

The Critical Role of the Vacuum Environment
Preventing Magnesium Oxidation
The most significant challenge in synthesizing Mg3Sb2 is the chemical nature of Magnesium. Magnesium is highly reactive and prone to rapid oxidation when exposed to oxygen at high temperatures.
A standard furnace environment would lead to the formation of impurities, degrading the material's final thermoelectric properties. The vacuum environment creates a barrier that isolates the melt from oxygen, preserving the purity of the Magnesium.
Facilitating Liquid-Phase Reactions
To form a proper compound, the raw elements must transition into a liquid state to interact at the atomic level. The furnace maintains a specific high temperature, typically around 1,173 K.
At this temperature, the solid Mg, Sb, and Sn elements melt completely. This liquid phase is essential for the components to undergo a full chemical reaction, resulting in a uniform and chemically stable alloy.
Achieving Material Homogeneity
Creating a Uniform Alloy
Thermoelectric performance relies heavily on the consistency of the material. The high-temperature vacuum process ensures that the synthesized ingot is homogeneous.
By allowing the elements to mix thoroughly in the liquid state, the furnace prevents phase separation. This ensures that the final solid material has a consistent composition throughout its volume.
Minimizing Gas Impurities
Beyond preventing oxidation, the vacuum atmosphere actively minimizes the inclusion of other gas impurities.
This results in a "cleaner" material structure. Reducing gas inclusions at this initial stage is vital for maintaining high electrical transport properties in the final device.
Distinguishing Synthesis from Densification
Understanding the Process Flow
It is critical not to confuse the synthesis furnace with the Vacuum Hot Pressing (VHP) furnace, as they serve different purposes in the production line.
The high-temperature vacuum furnace described above is used for the initial reaction (melting at ~1,173 K without pressure).
The Role of Vacuum Hot Pressing (VHP)
In contrast, VHP is used later for densification. It operates at lower temperatures (e.g., 873 K) but applies high mechanical pressure (e.g., 70 MPa).
While the synthesis furnace focuses on chemical formation and purity, the VHP furnace focuses on eliminating pores and maximizing mechanical density.
Making the Right Choice for Your Goal
To optimize your Mg3Sb2 production process, ensure you are applying the correct equipment to the correct stage of development:
- If your primary focus is Chemical Purity: Prioritize the vacuum high-temperature furnace to melt raw elements at 1,173 K, ensuring the Magnesium remains unoxidized during reaction.
- If your primary focus is Material Density: Utilize a Vacuum Hot Pressing (VHP) system after synthesis to apply pressure (70 MPa) and eliminate porosity.
- If your primary focus is Homogeneity: Ensure your synthesis furnace can maintain a stable 1,173 K temperature to allow for complete liquid-phase mixing before cooling.
Mastering the vacuum synthesis step is the foundation for achieving high-performance thermoelectric materials.
Summary Table:
| Feature | Synthesis Vacuum Furnace | Vacuum Hot Pressing (VHP) |
|---|---|---|
| Primary Goal | Chemical reaction & purity | Densification & pore elimination |
| Temperature | High (~1,173 K) | Moderate (~873 K) |
| Pressure | Vacuum only | High mechanical pressure (70 MPa) |
| Material State | Liquid-phase melting | Solid-state pressing |
| Key Outcome | Homogeneous oxide-free alloy | High-density thermoelectric pellet |
Elevate Your Material Synthesis with KINTEK
Precise thermal control and high-vacuum integrity are non-negotiable for reactive materials like Mg3Sb2. KINTEK provides industry-leading high-temperature vacuum systems designed to eliminate oxidation and ensure absolute chemical homogeneity in your research and production.
Our Advantage to You:
- Expert R&D & Manufacturing: Reliable systems optimized for specialized thermoelectric synthesis.
- Comprehensive Portfolio: From Muffle and Tube Furnaces to advanced Vacuum, CVD, and Rotary systems.
- Fully Customizable: Tailored thermal solutions to meet your specific temperature and atmospheric requirements.
Ready to achieve superior material purity? Contact our technical experts today to find the perfect furnace for your laboratory.
Visual Guide
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace Molybdenum Wire Vacuum Sintering Furnace
People Also Ask
- What are the benefits of using graphite felt in vacuum furnaces? Achieve Superior Thermal Efficiency & Stability
- How should one choose between a vacuum furnace and an atmosphere furnace? Select the Right Furnace for Your Process
- What is the range of carburizing temperatures in vacuum carburizing? Optimize for Speed and Quality
- What role do vacuum sintering furnaces play in additive manufacturing? Transform 3D Prints into Dense, High-Performance Parts
- What is the maximum vacuum level for a high vacuum furnace? Achieve Ultra-Clean Processing for Advanced Materials
- What is the primary use of a vacuum graphitizing furnace? Transforming Carbon into High-Performance Graphite
- How does a vacuum brazing furnace prevent oxidation during the heating process? Achieve Clean, Strong Joints with Oxidation-Free Brazing
- How do continuous furnaces demonstrate versatility in processing? Unlock Multi-Process Efficiency for High-Volume Manufacturing



















