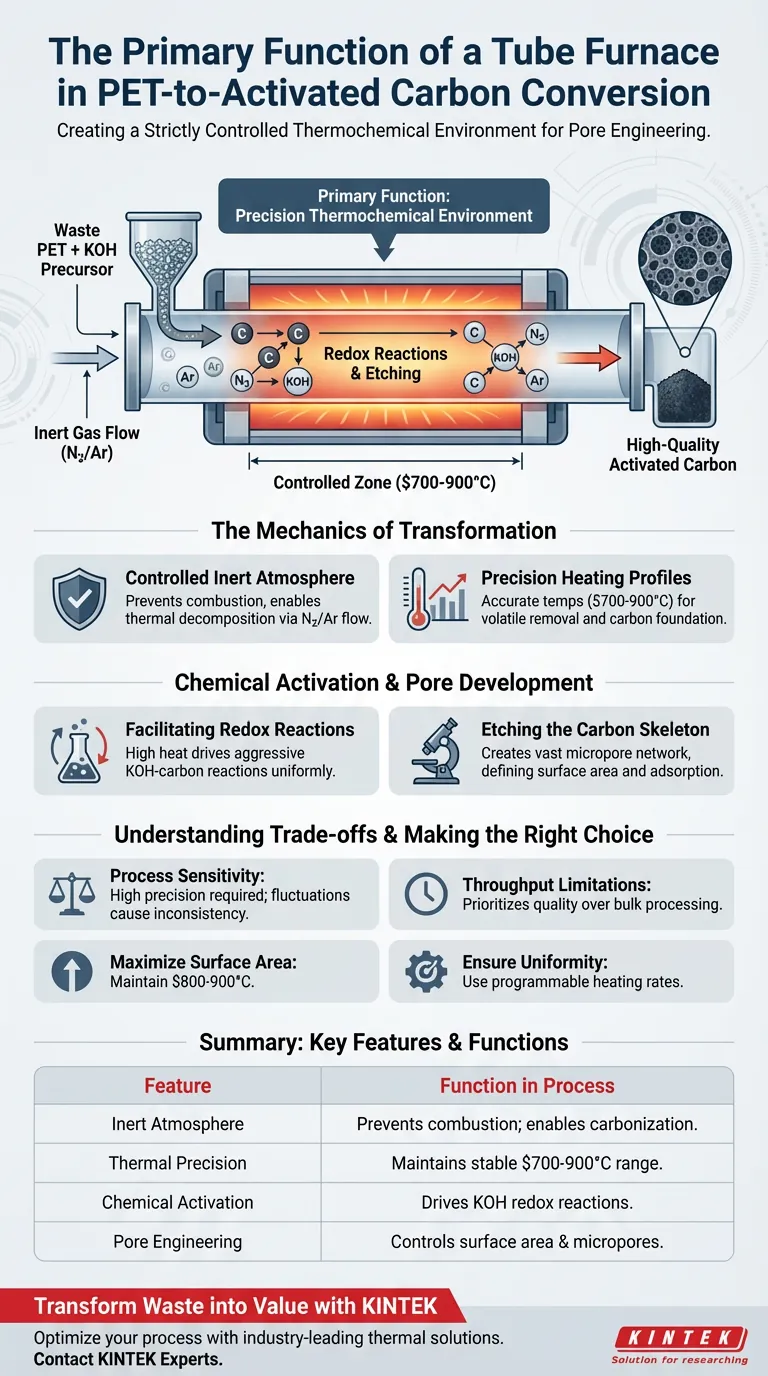
The primary function of a Tube Furnace in this context is to create a strictly controlled thermochemical environment that enables the transformation of waste PET into high-quality activated carbon. It achieves this by maintaining a precise high-temperature range ($700^\circ\text{C}$ to $900^\circ\text{C}$) while sustaining an inert atmosphere, typically using nitrogen or argon. This isolation allows for the dehydration, carbonization, and subsequent activation of chemically treated PET precursors without the risk of uncontrolled combustion.
Core Takeaway The Tube Furnace acts as the critical vessel for pore engineering, facilitating redox reactions between the carbon precursor and chemical activators like KOH. Its precision in controlling temperature and atmosphere directly dictates the final specific surface area and micropore structure of the activated carbon.
The Mechanics of Transformation
Controlled Inert Atmosphere
The most vital role of the Tube Furnace is the maintenance of a non-oxidizing environment. By continuously flowing inert gases such as nitrogen or argon through the tube, the system purges oxygen.
This prevents the PET material from burning away (oxidizing) as it would in an open fire. Instead, the inert environment forces the material to undergo thermal decomposition and carbonization, retaining the carbon structure needed for the final product.
Precision Heating Profiles
Converting PET requires specific thermal ramps and distinct temperature plateaus. The Tube Furnace is designed to reach temperatures between $700^\circ\text{C}$ and $900^\circ\text{C}$ with high accuracy.
Some processes utilize rapid heating rates, such as $80^\circ\text{C}/\text{min}$, to reach activation temperatures efficiently. This precise thermal control ensures the process remains stable enough to eliminate volatile organic compounds while establishing a solid carbon foundation.
Chemical Activation and Pore Development
Facilitating Redox Reactions
The furnace provides the energy required for chemical agents, specifically potassium hydroxide (KOH), to react with the pre-carbonized PET. The high heat drives redox reactions between the KOH and the carbon skeleton.
These reactions are aggressive and would be impossible to control without the uniform heat distribution provided by the tube design.
Etching the Carbon Skeleton
The ultimate goal of using this equipment is to develop porosity. As the KOH reacts under high heat, it effectively "etches" the carbon material.
This etching process creates a vast network of micropores. Consequently, the Tube Furnace is the core equipment responsible for determining the activated carbon's specific surface area and adsorption capacity.
Understanding the Trade-offs
Process Sensitivity
While Tube Furnaces offer exceptional control, they are highly sensitive to operational parameters. A fluctuation in gas flow or a deviation in the temperature ramp can lead to inconsistent activation or blocked pores.
Throughput Limitations
Tube furnaces are generally batch-processing or low-throughput continuous systems. They prioritize material quality and parameter precision over the high-volume bulk processing seen in large rotary kilns.
Making the Right Choice for Your Goal
To maximize the efficacy of a Tube Furnace in PET conversion, consider your specific end-goals:
- If your primary focus is Surface Area: Ensure your furnace can maintain the upper range of activation temperatures ($800^\circ\text{C}$–$900^\circ\text{C}$) to maximize the etching effect of the KOH.
- If your primary focus is Pore Structure Uniformity: Prioritize a furnace with programmable heating rates to control the speed of the redox reactions, preventing pore collapse.
Success in converting waste PET relies not just on the heat, but on the precision of the environment you build around it.
Summary Table:
| Feature | Function in PET-to-Carbon Process |
|---|---|
| Inert Atmosphere | Prevents combustion; enables dehydration and carbonization via N2/Ar flow. |
| Thermal Precision | Maintains 700°C–900°C for stable volatile removal and carbon structure development. |
| Chemical Activation | Provides thermal energy for KOH redox reactions to etch the carbon skeleton. |
| Pore Engineering | Directly controls specific surface area and micropore development through heat ramps. |
Transform Waste into Value with KINTEK
Ready to optimize your PET conversion process? KINTEK provides industry-leading thermal solutions backed by expert R&D and manufacturing. Whether you need a standard setup or a customized system for unique research goals, our range of Tube, Muffle, Rotary, Vacuum, and CVD systems offers the precision required for high-performance activated carbon production.
Maximize your lab's efficiency and material quality today.
Visual Guide
References
- Kiran Kumar Reddy Reddygunta, Aruna Ivaturi. Scalable slot-die coated flexible supercapacitors from upcycled PET face shields. DOI: 10.1039/d2ra06809e
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Controlled Inert Nitrogen Atmosphere Furnace
People Also Ask
- What is the difference between a vacuum tube furnace and a standard tube furnace? Choose the Right Furnace for Your Lab
- What is the function of a high-temperature tube furnace in Ti3AlC2 synthesis? Achieve Pure MAX Phase Precursor Powders
- What critical role does a laboratory tube furnace play in pBN-CTF synthesis? Master Molecular Engineering
- What is the significance of a vacuum tube furnace system? Master Reaction Rate Constants for Carbonate Thin Films
- Why is a secondary high-temperature activation process in a tube furnace necessary? Converting Biochar into CBAC
- How does an electric heating tube furnace ensure stable experimental conditions? Master Bio-Oil Upgrading Stability
- How does a manual laboratory jack contribute to process precision in split tube furnaces? Achieve Perfect Alignment
- What are the critical functions of a laboratory tube furnace in biomass synthesis? Optimize Your Carbonization Process



















