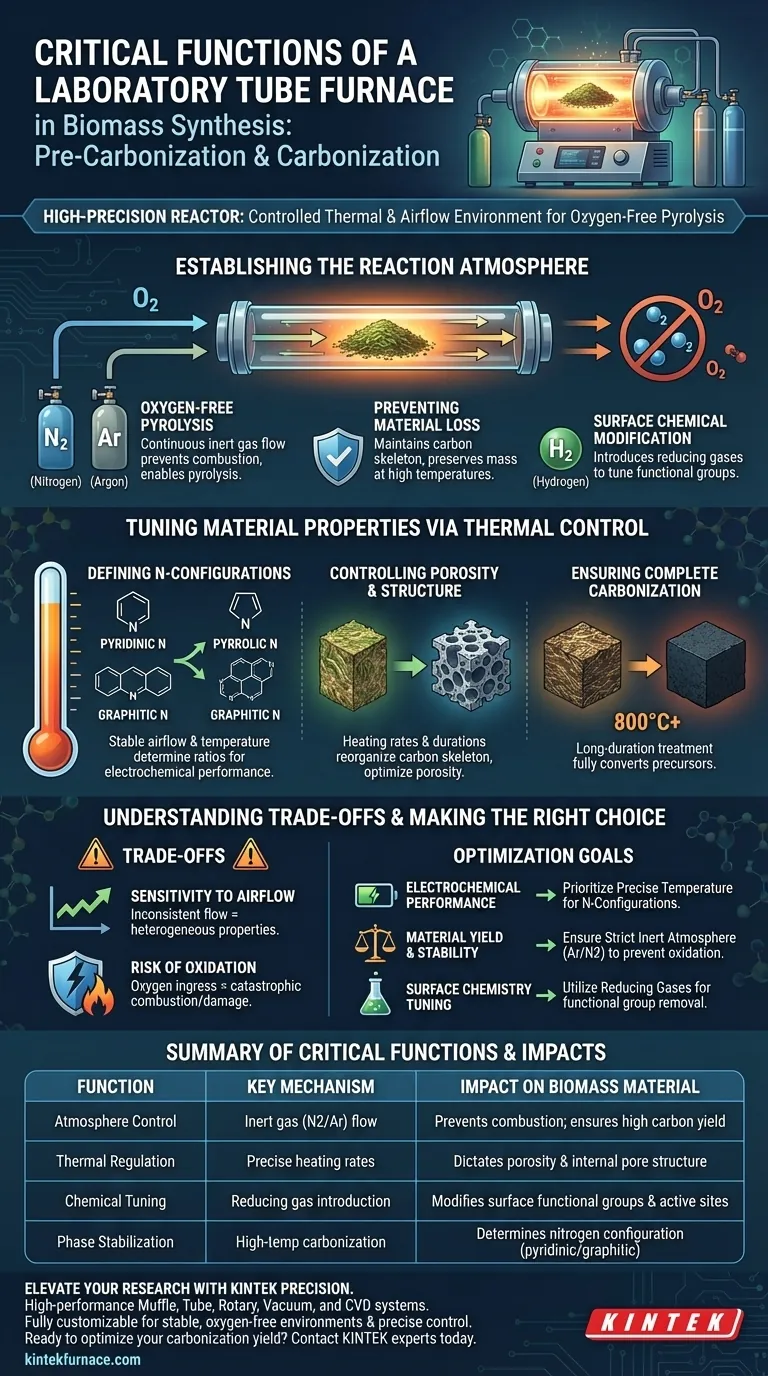
A laboratory tube furnace functions as a high-precision reactor during biomass synthesis, serving as far more than a simple heating element. Its critical role is to provide a strictly controlled thermal treatment environment and stable airflow management, typically utilizing inert gases like nitrogen or argon to facilitate oxygen-free pyrolysis.
The laboratory tube furnace is the defining instrument for tailoring the microstructure and surface chemistry of biomass-derived materials. By coupling precise temperature regulation with specific gas atmospheres, it determines the final material's porosity, elemental ratios, and atomic-level configuration.

Establishing the Reaction Atmosphere
The most immediate function of the tube furnace is to isolate the biomass from atmospheric oxygen.
Oxygen-Free Pyrolysis
The furnace introduces a continuous flow of inert gases, such as nitrogen or argon. This creates an oxygen-free environment that is essential for converting biomass into carbon (pyrolysis) rather than burning it away through combustion.
Preventing Material Loss
By maintaining a strict inert atmosphere, the furnace prevents the excessive oxidation of the carbon skeleton at high temperatures. This preservation of mass is crucial for ensuring a viable yield of conductive carbon layers and stabilizing internal crystalline phases.
Surface Chemical Modification
Beyond inert gases, the furnace can introduce reducing gases, such as hydrogen mixtures. This facilitates the directional removal of specific oxygen-containing functional groups, allowing researchers to adjust the chemical properties of catalytic active sites without damaging the material's pore structure.
Tuning Material Properties via Thermal Control
The precise regulation of heat and airflow directly dictates the physical and chemical identity of the synthesized material.
Defining Nitrogen Configurations
The stability of the airflow and temperature controls are the primary factors determining the specific ratios of nitrogen configurations. The furnace environment dictates the formation of pyridinic, pyrrolic, and graphitic nitrogen, which are critical for the material's electrochemical performance.
Controlling Porosity and Structure
Accurate control over heating rates and constant temperature durations allows for the systematic removal of volatile components. This process reorganizes the carbon skeleton, resulting in a hard carbon structure with high specific capacity and optimized porosity.
Ensuring Complete Carbonization
The furnace enables long-duration thermal treatments at high temperatures (e.g., 800 °C). This ensures that precursors, such as ZIFs or pre-oxidized bark, are completely carbonized into heteroatom-doped materials with specific active sites.
Understanding the Trade-offs
While the tube furnace enables precision, it requires rigorous management of operational variables to avoid common pitfalls.
Sensitivity to Airflow Fluctuations
The quality of the final material is heavily dependent on stable airflow management. Inconsistent gas flow can lead to uneven doping of nitrogen or incomplete removal of volatiles, resulting in heterogeneous material properties.
The Risk of Oxidation
The system's reliance on a "strictly controlled" environment means any breach in the inert atmosphere is catastrophic. Even minor oxygen ingress during the high-temperature phase can lead to the combustion of the biomass or the destruction of the desired pore structure.
Making the Right Choice for Your Goal
To maximize the utility of a laboratory tube furnace for biomass synthesis, align your operational parameters with your specific material targets.
- If your primary focus is Electrochemical Performance: Prioritize precise temperature control to dictate specific nitrogen configurations (pyridinic vs. graphitic) which act as active sites.
- If your primary focus is Material Yield and Stability: Ensure a strict, high-purity inert atmosphere (Argon/Nitrogen) to prevent oxidation and preserve the carbon skeleton.
- If your primary focus is Surface Chemistry Tuning: Utilize the furnace's ability to introduce reducing gases to selectively remove oxygen functional groups without collapsing pores.
Success in biomass carbonization relies not just on reaching high temperatures, but on the absolute stability of the thermal and atmospheric environment provided by the furnace.
Summary Table:
| Function | Key Mechanism | Impact on Biomass Material |
|---|---|---|
| Atmosphere Control | Inert gas (N2/Ar) flow | Prevents combustion; ensures high carbon yield |
| Thermal Regulation | Precise heating rates | Dictates porosity and internal pore structure |
| Chemical Tuning | Reducing gas introduction | Modifies surface functional groups and active sites |
| Phase Stabilization | High-temp carbonization | Determines nitrogen configuration (pyridinic/graphitic) |
Elevate Your Material Research with KINTEK Precision
Don't let inconsistent airflow or temperature fluctuations compromise your biomass synthesis. KINTEK provides high-performance laboratory solutions, including Muffle, Tube, Rotary, Vacuum, and CVD systems, specifically engineered for rigorous pre-carbonization and carbonization stages.
Backed by expert R&D and manufacturing, our furnaces are fully customizable to meet your unique research needs, ensuring stable oxygen-free environments and precise thermal control for superior electrochemical performance.
Ready to optimize your carbonization yield? Contact KINTEK experts today for a custom solution
Visual Guide
References
- Xing Huang, Dessie Ashagrie Tafere. Waste-derived green N-doped materials: mechanistic insights, synthesis, and comprehensive evaluation. DOI: 10.1039/d5su00555h
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Laboratory Quartz Tube Furnace RTP Heating Tubular Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
People Also Ask
- How does a high-temperature tube furnace facilitate the ammonolysis process? Master TiNx Nanoparticle Synthesis
- What is a multi zone tube furnace used for? Unlock Precision Thermal Processing for Advanced Materials
- What are some examples of research applications for lab tubular furnaces? Unlock Precision in High-Temperature Experiments
- How does the design of a dual-zone Tube Furnace facilitate precise metal phosphide conversion? Optimize Heterojunctions
- What safety precautions should be followed when operating a multi zone tube furnace? Ensure Safe and Efficient Lab Operations
- What are some advanced features of more elaborate tube furnaces? Unlock Precision Control for High-Temp Processes
- What components are used in tube furnaces to achieve temperatures above 1200 °C? Key Elements for Extreme Heat
- How does a horizontal tube furnace work? Master Precise Thermal Processing for Your Lab



















