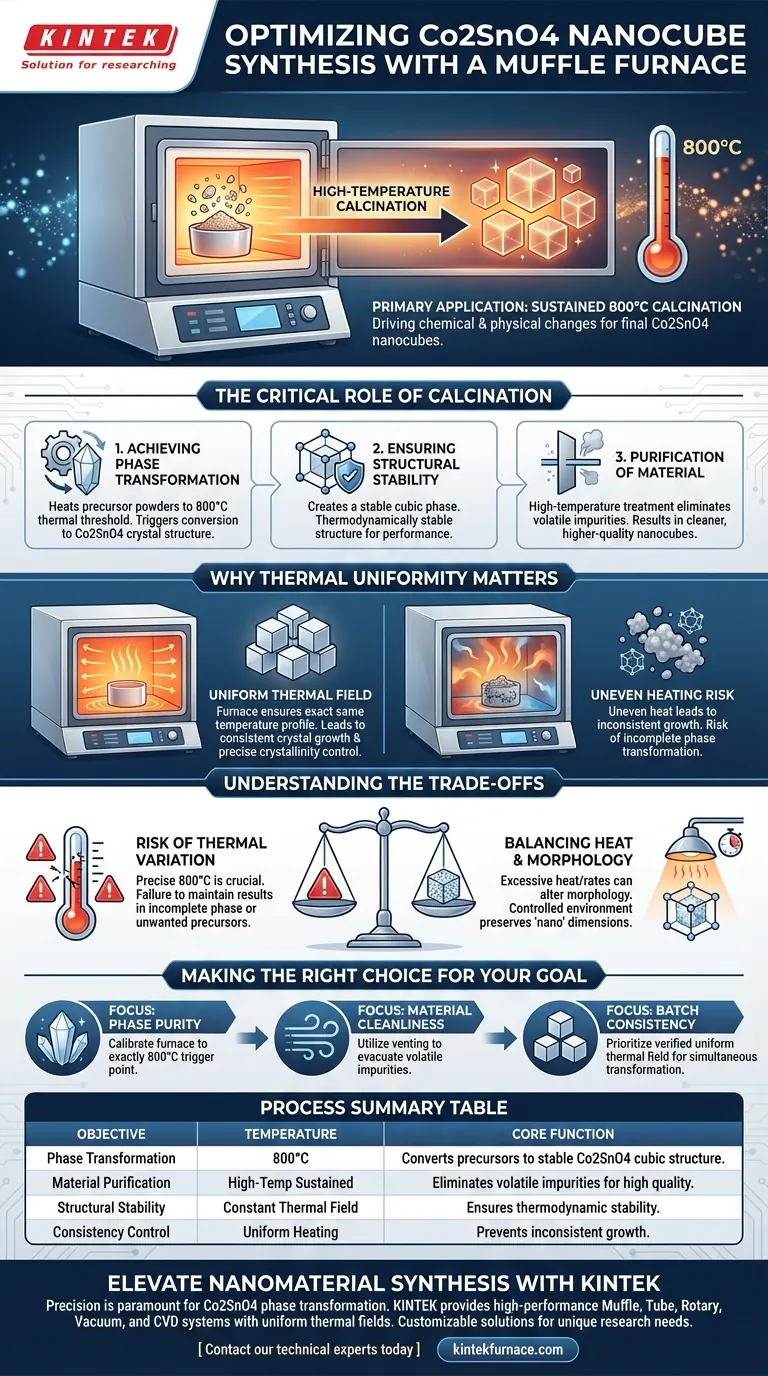
In the preparation of Co2SnO4 nanocubes, the laboratory muffle furnace is utilized primarily for the high-temperature calcination of precursor powders. Specifically, it subjects the material to a sustained temperature of 800°C to drive the chemical and physical changes necessary to finalize the nanomaterial.
The muffle furnace provides a uniform thermal field that is essential for transforming the precursor into a stable cubic crystal structure and effectively eliminating volatile impurities.
The Critical Role of Calcination
Achieving Phase Transformation
The central purpose of the muffle furnace in this workflow is to facilitate a specific phase change.
The precursor powders must be heated to 800°C. At this distinct thermal threshold, the energy triggers a transformation, converting the raw precursors into the desired Co2SnO4 crystal structure.
Ensuring Structural Stability
The output of this process is not just any crystal, but a stable cubic phase.
The controlled environment of the muffle furnace ensures that this cubic structure is thermodynamically stable. This stability is the foundation for the material's performance in subsequent applications.
Purification of the Material
Beyond structural formation, the furnace serves as a purification tool.
During the synthesis process, various volatile impurities are often trapped within the material. The high-temperature treatment effectively removes these contaminants, resulting in a cleaner, higher-quality nanocube product.
Why Thermal Uniformity Matters
The Importance of the Thermal Field
A laboratory muffle furnace is chosen over other heating methods because of its ability to generate a uniform thermal field.
In nanomaterial preparation, uneven heating can lead to inconsistent crystal growth. The muffle furnace ensures that every part of the sample experiences the exact same temperature profile.
Impact on Crystallinity
The quality of the final nanocube is directly tied to the consistency of the heat applied.
By maintaining a static and uniform environment, the furnace allows for precise control over the crystallinity of the Co2SnO4. This ensures that the physical properties of the batch are homogenous.
Understanding the Trade-offs
The Risk of Thermal Variation
While muffle furnaces are designed for uniformity, the specific parameters used (such as the 800°C target) leave little room for error.
If the furnace fails to maintain this precise temperature or if the thermal field becomes uneven, the phase transformation may be incomplete. This can result in a material that lacks the intended cubic structure or retains unwanted precursor phases.
Balancing Heat and Morphology
There is a delicate balance between achieving the necessary phase transformation and maintaining the "nano" dimensions of the material.
High-temperature calcination promotes crystallization, but excessive heat or uncontrolled ramp rates can potentially alter the morphology or cause agglomeration. The process relies heavily on the furnace's ability to hold the 800°C setpoint accurately without overshooting.
Making the Right Choice for Your Goal
When configuring your thermal treatment for Co2SnO4 preparation, consider the following:
- If your primary focus is Phase Purity: Ensure your furnace is calibrated to maintain exactly 800°C, as this is the trigger point for forming the stable cubic Co2SnO4 structure.
- If your primary focus is Material Cleanliness: Utilize the furnace's venting or static air capabilities to ensure volatile impurities generated during synthesis are fully evacuated.
- If your primary focus is Batch Consistency: Prioritize a furnace with a verified uniform thermal field to guarantee that all precursor powder undergoes the same phase transformation simultaneously.
Mastering the calcination step is the key to converting raw chemical potential into a robust, high-performance nanomaterial.
Summary Table:
| Process Objective | Temperature Requirement | Core Function of Muffle Furnace |
|---|---|---|
| Phase Transformation | 800°C | Converts precursors into stable Co2SnO4 cubic crystal structures. |
| Material Purification | High-Temp Sustained | Eliminates volatile impurities to ensure high-quality nanomaterials. |
| Structural Stability | Constant Thermal Field | Ensures thermodynamic stability for uniform nanocube performance. |
| Consistency Control | Uniform Heating | Prevents inconsistent crystal growth and incomplete phase changes. |
Elevate Your Nanomaterial Synthesis with KINTEK
Precision is paramount when managing the delicate phase transformation of Co2SnO4 nanocubes. Backed by expert R&D and world-class manufacturing, KINTEK provides high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to deliver the thermal uniformity your lab requires. Whether you need a standard solution or a system fully customizable for unique research needs, our furnaces ensure your materials reach their full potential.
Ready to optimize your high-temperature processes?
Contact our technical experts today to find the perfect furnace for your laboratory applications.
Visual Guide
References
- Nitrogen-Doped Hollow Carbon Spheres-Decorated Co2SnO4/WS2 Heterostructures with Improved Visible-Light Photocatalytic Degradation of Organic Dye. DOI: 10.3390/molecules30092081
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- How are box furnaces utilized in electronic component manufacturing? Essential Thermal Processing for Electronics
- What is a muffle furnace and what are its primary uses? Unlock Precise High-Temp Solutions
- How does the high-temperature calcination process in a muffle furnace facilitate the structural transformation of KMnPO4·H2O?
- How does a muffle furnace contribute to the post-processing of SnO2? Engineering Superior Nanoparticle Crystallinity
- What are the installation and maintenance benefits of electric furnaces? Achieve Simpler, Lower-Cost Heating
- What are the common applications of box furnaces? Versatile Heat Treatment for Metals, Ceramics, and Research
- What is the difference between a box furnace and a muffle furnace? Understand Key Design Principles
- How do you prevent maintenance on a muffle furnace? Extend Lifespan with Proactive Care



















