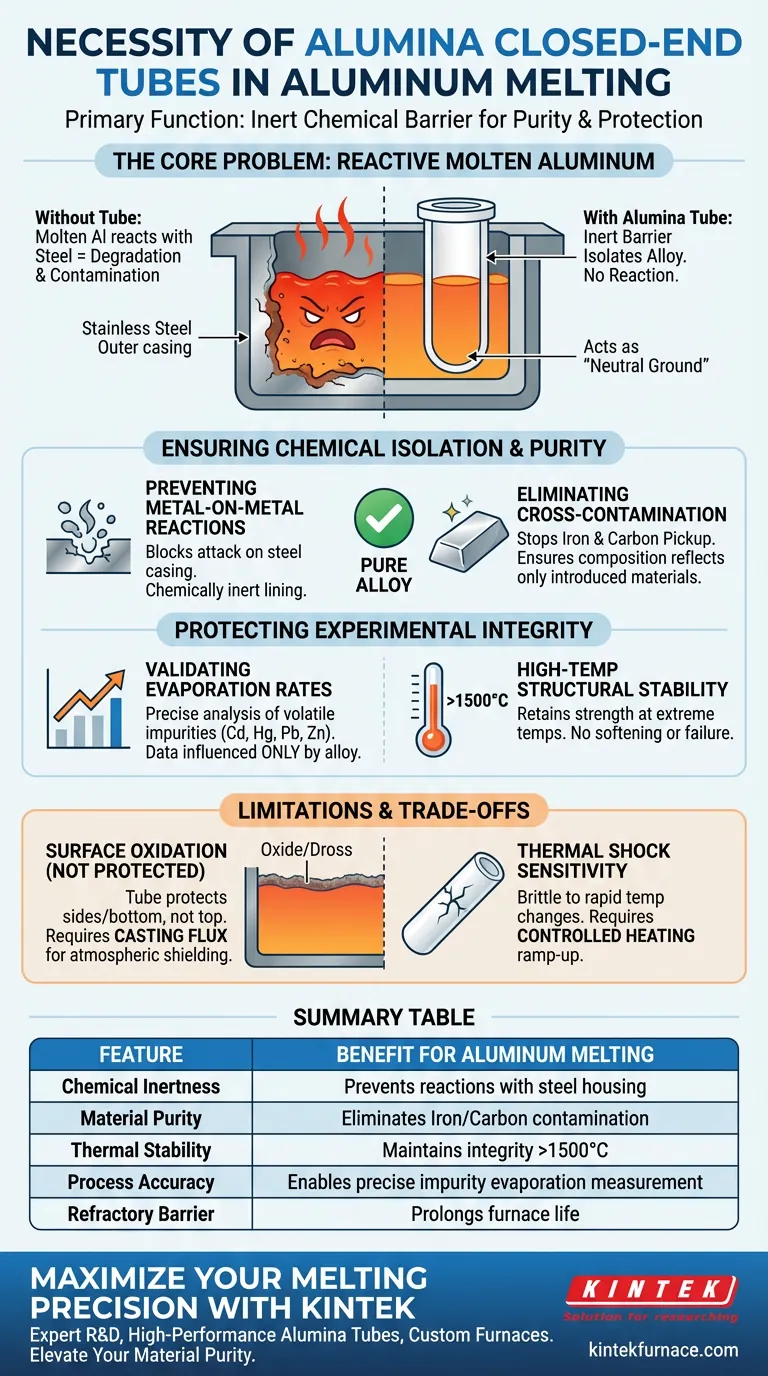
The primary necessity of using an alumina closed-end tube is to function as an inert chemical barrier. It physically isolates the reactive molten aluminum alloy from the furnace's outer stainless steel casing. Without this refractory lining, the molten aluminum would react with the steel housing, leading to equipment degradation and severe contamination of your alloy.
The alumina tube acts as a "neutral ground" within the furnace. By preventing the molten alloy from touching the external metal hardware, it guarantees that the material’s composition remains pure and that any changes in the melt are driven solely by physics, not by reactions with the container.

Ensuring Chemical Isolation and Purity
Preventing Metal-on-Metal Reactions
Molten aluminum is chemically aggressive and a universal solvent for many metals. If allowed to touch the outer stainless steel casing, it will attack and dissolve the steel.
The alumina (aluminum oxide) tube serves as a chemically inert lining. It prevents the high-temperature chemical reactions that would otherwise occur between the liquid alloy and the furnace walls.
Eliminating Cross-Contamination
When molten alloys interact with furnace hardware, carbonization and iron contamination are common side effects.
The alumina tube blocks these contaminants. This isolation ensures that the final chemical composition of your ingot reflects only the materials you introduced, not the materials of the furnace itself.
Protecting Experimental Integrity
Validating Evaporation Rates
In precise metallurgical processes, you may be tracking the behavior of volatile impurities such as cadmium (Cd), mercury (Hg), lead (Pb), and zinc (Zn).
The primary reference indicates that the alumina tube is critical for this analysis. By eliminating hardware interactions, you ensure that the evaporation rates of these elements are influenced only by the alloy's composition and physical conditions, rather than by external contamination variables.
High-Temperature Structural Stability
Alumina is a refractory material, meaning it retains its strength at extreme temperatures.
According to supplementary data, alumina maintains structural integrity in environments exceeding 1500°C. This thermal stability ensures the container does not soften, deform, or fail during the melting process, which is critical for safety and process reliability.
Understanding the Limitations and Trade-offs
The "Container vs. Surface" Distinction
While the alumina tube protects the sides and bottom of the melt, it does not protect the top surface from the atmosphere.
To fully protect the alloy—especially those high in magnesium or zinc—you must still address surface oxidation. This often requires the use of casting flux to form a physical barrier on the melt pool, preventing hydrogen absorption and inclusion formation. The tube handles containment; the flux handles atmospheric shielding.
Thermal Shock Sensitivity
Alumina is excellent at resisting heat, but it can be brittle if temperature changes are too rapid.
Unlike metal crucibles, alumina tubes generally require a controlled heating ramp-up. Rapid heating or cooling can cause the ceramic to crack due to thermal shock, potentially breaching the containment you are trying to establish.
Making the Right Choice for Your Goal
To ensure your melting process produces high-quality results, assess your specific requirements:
- If your primary focus is experimental accuracy: Rely on the alumina tube to ensure that data regarding impurity evaporation (like Zn or Pb) is statistically valid and unaffected by the vessel.
- If your primary focus is alloy purity: Use the tube to prevent iron pickup from the steel shell, but pair it with a surface flux to prevent oxide inclusions.
- If your primary focus is equipment longevity: Inspect the alumina tube regularly for hairline cracks, as its failure will immediately expose your expensive stainless steel housing to liquid aluminum attack.
By using an alumina closed-end tube, you transform a chaotic chemical environment into a controlled vessel, ensuring the physics of your melt take precedence over the chemistry of your equipment.
Summary Table:
| Feature | Benefit for Aluminum Melting |
|---|---|
| Chemical Inertness | Prevents reactions between molten aluminum and steel housing |
| Material Purity | Eliminates iron and carbon contamination for high-grade alloys |
| Thermal Stability | Maintains structural integrity at temperatures exceeding 1500°C |
| Process Accuracy | Enables precise measurement of volatile impurity evaporation (Zn, Pb) |
| Refractory Barrier | Prolongs furnace life by shielding outer metal components |
Maximize Your Melting Precision with KINTEK
Don’t let equipment contamination compromise your metallurgical results. Backed by expert R&D and manufacturing, KINTEK offers high-performance Alumina tubes and a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. Whether you need standard lab high-temp furnaces or fully customizable solutions tailored to your unique research needs, we deliver the thermal expertise you require.
Ready to elevate your material purity? Contact us today to consult with our technical team!
Visual Guide
References
- Aleksandar M. Mitrašinović, Milinko Radosavljević. Modeling of Impurities Evaporation Reaction Order in Aluminum Alloys by the Parametric Fitting of the Logistic Function. DOI: 10.3390/ma17030728
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the role of an infrared pyrometer in wood carbonization? Optimize Your High-Temp Thermal Control
- What is the function of the circulating water cooling system? Optimize Pyrolysis Oil Condensation and Yield
- Why use high-purity graphite for β-Ga2O3 annealing? Key to Thermal Precision & Safety
- What function do high-strength graphite molds serve? Essential Roles in Ti-6Al-4V Vacuum Hot Pressing
- How does an oil-free rotary vane vacuum pump contribute to aluminum powder processing? Ensure Purity & Stability
- What are the primary functions of multilayer fixtures within a lithium battery vacuum oven? Optimize Your Drying Process
- What is the function of a ceramic crucible with a lid during g-C3N4 synthesis? Optimize Your Polycondensation Results
- How are quartz tubes applied in optics and pharmaceuticals? Unlock Purity and Performance in Critical Applications



















