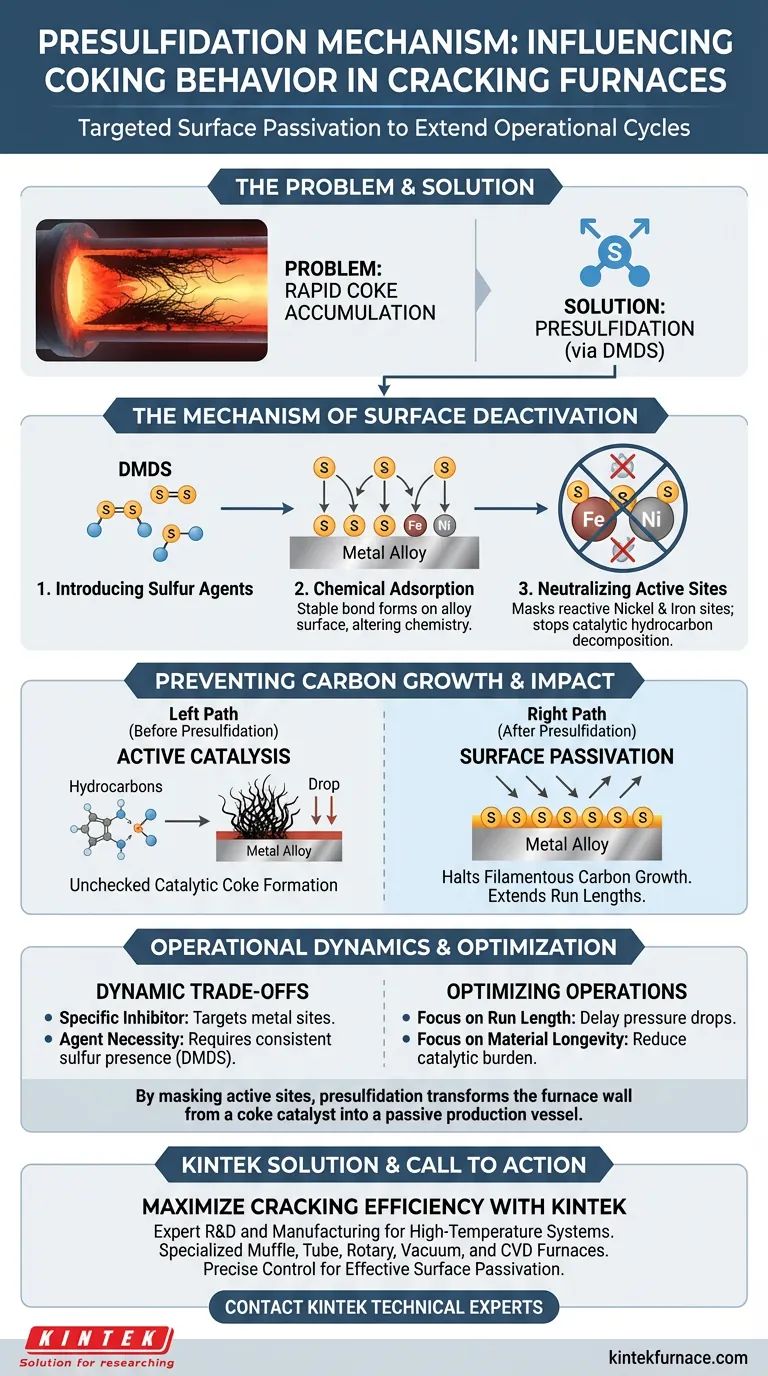
Presulfidation functions as a targeted surface passivation technique. It influences coking behavior by introducing sulfur compounds, such as dimethyl disulfide (DMDS), into the furnace system. These compounds chemically interact with the alloy surface to mask catalytic sites, effectively preventing the initial reactions that lead to rapid coke accumulation.
By chemically adsorbing sulfur atoms onto reactive metals like nickel and iron, presulfidation neutralizes the surface's catalytic activity. This disrupts the specific mechanism responsible for filamentous carbon growth, significantly extending the operational cycle of cracking furnaces.
The Mechanism of Surface Deactivation
To understand how presulfidation extends furnace run lengths, one must look at the interaction between the sulfur agent and the metallurgy of the tube.
Introducing Sulfur Agents
The process begins with the introduction of a sulfur source into the cracking system.
Common agents, such as dimethyl disulfide (DMDS), act as the delivery vehicle for the necessary sulfur atoms.
Chemical Adsorption
Once introduced, the sulfur does not merely coat the surface; it undergoes chemical adsorption.
This creates a stable bond between the sulfur atoms and the metal surface, altering the surface chemistry of the material.
Neutralizing Active Sites
The primary targets of this adsorption are specific metal atoms within the alloy, particularly nickel and iron.
These metals are naturally reactive and, without intervention, act as "active sites" that facilitate unwanted chemical reactions.
Preventing Carbon Growth
The ultimate goal of deactivating these metal sites is to interrupt the physical growth of coke deposits.
Halting Catalytic Activity
Nickel and iron atoms on the tube surface naturally catalyze the decomposition of hydrocarbons.
By covering these atoms with sulfur, presulfidation disrupts their ability to catalyze this breakdown, effectively turning off the "engine" of coke formation at the wall.
Blocking Filamentous Carbon
The specific result of this catalytic disruption is the inhibition of filamentous carbon formation.
Filamentous carbon is a rapid-growth form of coke that limits run lengths; preventing its formation is critical for extending cracking operation cycles in both industrial and laboratory contexts.
Operational Dynamics and Trade-offs
While presulfidation is effective, it relies on precise chemical interactions.
Specificity of the Inhibitor
The process is highly specific to the deactivation of metal sites.
It functions by competing with carbon for access to nickel and iron atoms, necessitating a consistent presence of the passivating layer.
The Necessity of the Agent
The mechanism is entirely dependent on the successful introduction of the sulfur source (e.g., DMDS).
Without the chemical adsorption of sulfur, the metal sites remain active, and the catalytic formation of filamentous carbon will proceed unchecked.
Optimizing Cracking Operations
To maximize the lifespan of your furnace tubes and the duration of your run cycles, consider how this mechanism aligns with your operational goals.
- If your primary focus is extending run length: Ensure your presulfidation process effectively targets filamentous carbon formation to delay the onset of pressure drop limitations.
- If your primary focus is material longevity: Utilize sulfur sources to passivate nickel and iron sites, reducing the catalytic burden on the tube metallurgy.
By strategically masking active metal sites, presulfidation transforms the furnace wall from a catalyst for coke into a passive vessel for production.
Summary Table:
| Mechanism Stage | Process Action | Impact on Coking Behavior |
|---|---|---|
| Introduction | Delivery of DMDS sulfur agents | Prepares the surface for chemical interaction |
| Adsorption | Sulfur atoms bond to alloy surface | Masks reactive nickel and iron active sites |
| Deactivation | Neutralizes catalytic activity | Halts the decomposition of hydrocarbons at the wall |
| Inhibition | Blocking filamentous carbon growth | Prevents rapid coke accumulation and pressure drops |
| Result | Surface passivation | Significantly extends operational run cycles |
Maximize Your Cracking Efficiency with KINTEK
Don't let catalytic coking compromise your furnace performance. Backed by expert R&D and manufacturing, KINTEK offers specialized Muffle, Tube, Rotary, Vacuum, and CVD systems designed to withstand the rigors of high-temperature processing. Whether you need a standard laboratory furnace or a custom-engineered solution for your unique materials research, our systems provide the precise control required for effective surface passivation and thermal processing.
Ready to extend your furnace run lengths? Contact our technical experts today to discover how KINTEK’s customizable high-temperature solutions can optimize your production cycle.
Visual Guide
References
- Hamed Mohamadzadeh Shirazi, Kevin M. Van Geem. Effect of Reactor Alloy Composition on Coke Formation during Butane and Ethane Steam Cracking. DOI: 10.1021/acs.iecr.3c03180
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Magnesium Extraction and Purification Condensing Tube Furnace
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- How does the design and heating method of a tubular reactor facilitate simulated ethane cracking experiments?
- Why is a tube reduction furnace used for the pre-reduction of CeAlOx/NiO/Ni-foam catalysts? Essential Catalyst Prep
- What are the technical advantages of using a high-precision atmosphere tube furnace? Master Sensitive Ceramic Sintering
- What is the orientation referred to by the term 'horizontal' in horizontal tube furnaces? Optimize Your Thermal Processing with Expert Insights
- What are the types of Tube Furnaces based on tube shape? Choose Between Solid and Split for Your Lab
- Why is a tube furnace equipped with an atmosphere control system required for synthesizing h-Zn-Co-O solid solutions?
- Why is a Horizontal Tube Diffusion Furnace used for polysilicon doping? Master POCl3 Diffusion & Sheet Resistance
- Why is a tube furnace with precise temperature control necessary for Fe7S8@CT-NS composites? Master Advanced Synthesis



















