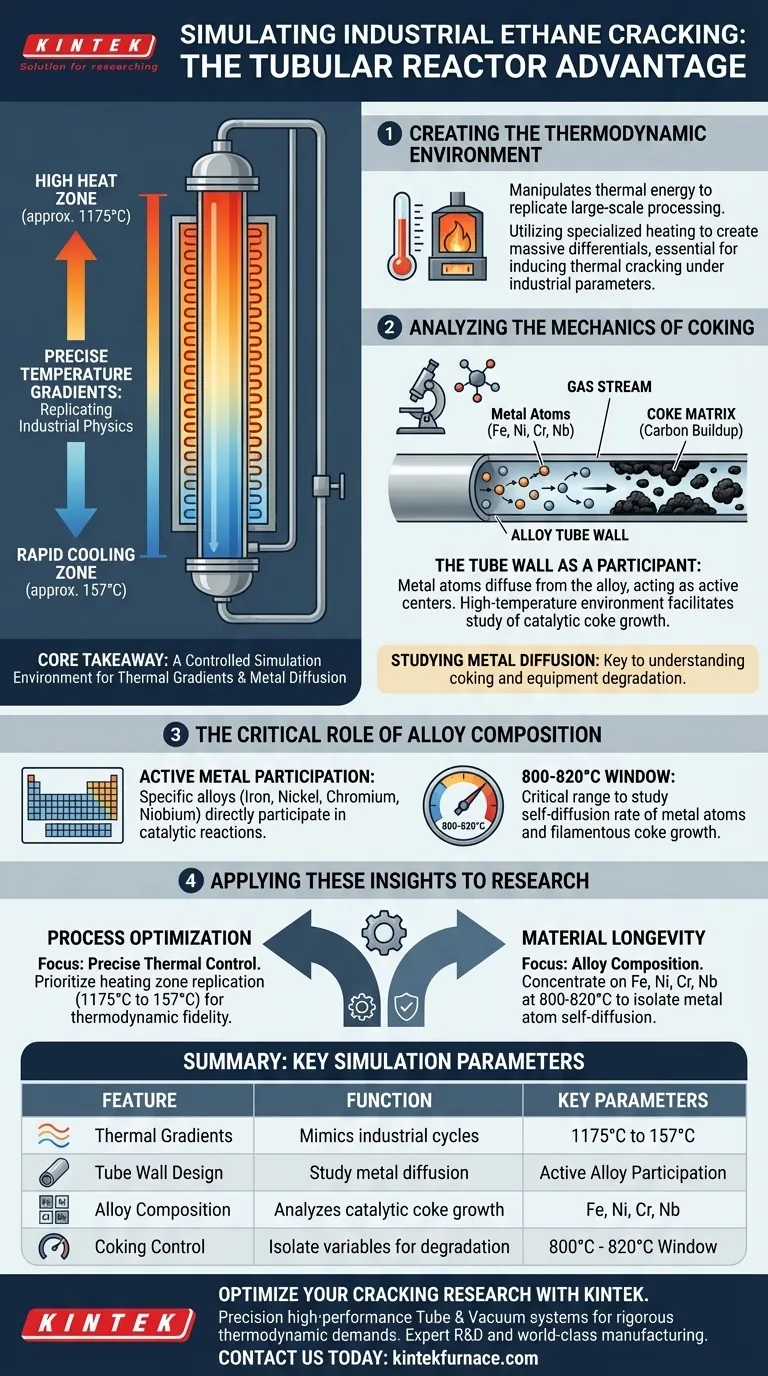
The design of a tubular reactor facilitates simulated ethane cracking by establishing a precise, highly variable heating zone that accurately mimics the extreme thermodynamic conditions of industrial production. By utilizing steep temperature gradients—ranging significantly from highs like 1175°C down to 157°C—the apparatus provides the specific physical environment required to induce thermal cracking while simultaneously isolating the variables necessary to study coke formation.
Core Takeaway: The tubular reactor is not merely a heating vessel; it is a controlled simulation environment. Its primary function is to replicate industrial thermal gradients to study how metal atoms diffuse from tube walls, enabling researchers to understand the catalytic mechanisms that drive coking and equipment degradation.

Creating the Thermodynamic Environment
The fundamental capability of the tubular reactor lies in its ability to manipulate thermal energy to replicate large-scale processing conditions.
Precise Temperature Gradients
The reactor utilizes a specialized heating zone designed to create massive temperature differentials.
References indicate gradients spanning from approximately 1175°C to 157°C.
This broad range is critical for simulating the rapid heating and cooling cycles found in industrial ethane cracking units.
Replicating Industrial Physics
This thermal structure provides the necessary thermodynamic conditions for reaction gases to undergo thermal cracking.
By strictly controlling this environment, researchers can ensure that the chemical breakdown of ethane occurs under parameters that align with real-world production data.
Analyzing the Mechanics of Coking
Beyond simple heating, the reactor's design is instrumental in studying the interaction between the reactor materials and the gas stream.
The Tube Wall as a Participant
The reactor design acknowledges that the tube wall is not a passive container.
It provides a physical space where metal atoms can diffuse from the alloy material.
This feature allows researchers to observe how the reactor material itself contributes to the reaction.
Studying Metal Diffusion
The high-temperature environment facilitates the study of how metal active centers migrate into the coke matrix.
This diffusion is a primary driver for inducing coking (carbon buildup) on the reactor walls.
The setup allows for the isolation of these variables to see specifically how wall materials degrade over time.
The Critical Role of Alloy Composition
While the heating method drives the reaction, the specific composition of the reactor tube defines the chemical interactions.
Active Metal Participation
The tubes are often composed of specific alloys containing iron, nickel, chromium, and niobium.
These elements are not inert; they participate directly in catalytic reactions during the cracking process.
Catalytic Influence on Coke Growth
In high-temperature windows (specifically around 800-820°C), the alloy composition becomes a critical variable.
Researchers use this specific thermal range to study the self-diffusion rate of metal atoms.
This helps identify how these metal centers induce the growth of filamentous coke, a major cause of reactor fouling.
Critical Considerations in Simulation
When utilizing a tubular reactor for these experiments, it is vital to understand the complexities involved in the simulation.
Sensitivity to Temperature Profiles
The simulation is highly sensitive to the exact temperature profile applied.
A deviation in the heating zone can alter the rate of metal atom diffusion.
This can lead to inaccurate data regarding how quickly coking will occur in a full-scale industrial unit.
Material Selection Variables
The specific ratio of alloy elements (e.g., 37:35:25:3 wt%) fundamentally changes the catalytic behavior.
Results derived from one specific alloy composition cannot be universally applied to reactors using different metallurgies.
Applying These Insights to Research
To maximize the value of simulated ethane cracking experiments, align your reactor setup with your specific research goals.
- If your primary focus is Process Optimization: Prioritize the precise control of the heating zone to replicate the 1175°C to 157°C gradient, ensuring thermodynamic fidelity to industrial standards.
- If your primary focus is Material Longevity: Concentrate on the alloy composition (Fe, Ni, Cr, Nb) and maintain temperatures in the 800-820°C range to isolate and measure the self-diffusion of metal atoms into the coke matrix.
By controlling both the thermal gradient and the metallurgical environment, you transform the reactor from a simple heater into a precise analytical tool for predicting industrial performance.
Summary Table:
| Feature | Function in Ethane Cracking Simulation | Key Parameters |
|---|---|---|
| Thermal Gradients | Mimics industrial heating/cooling cycles | 1175°C to 157°C |
| Tube Wall Design | Facilitates study of metal atom diffusion | Active Alloy Participation |
| Alloy Composition | Analyzes catalytic growth of filamentous coke | Fe, Ni, Cr, Nb |
| Coking Control | Isolate variables for equipment degradation | 800°C - 820°C Window |
Optimize Your Cracking Research with KINTEK
Precision is the difference between a simple experiment and a scalable industrial breakthrough. KINTEK provides high-performance, customizable Tube and Vacuum systems designed to meet the rigorous thermodynamic demands of ethane cracking simulations.
Backed by expert R&D and world-class manufacturing, our lab high-temp furnaces offer the exact thermal control needed to study metal diffusion and catalytic coking mechanisms.
Ready to elevate your material research? Contact us today to discuss your unique reactor needs and discover how our specialized equipment can bring industrial-grade accuracy to your laboratory.
Visual Guide
References
- P. Nanthagopal R. Sachithananthan. Analytical Review on Impact of Catalytic Coke Formation on Reactor Surfaces During the Thermal Cracking Process. DOI: 10.5281/zenodo.17985550
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- High Pressure Laboratory Vacuum Tube Furnace Quartz Tubular Furnace
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- Vertical Laboratory Quartz Tube Furnace Tubular Furnace
People Also Ask
- What core functions does a high-temperature tube furnace provide? Master TiN-Carbon Composite Pyrolysis
- What type of processing environment do high-temperature tube and muffle furnaces provide? Master Thermal Precision
- What is the role of a laboratory tube annealing furnace in LiMn2O4 coatings? Expert Post-Treatment Insights
- Why is calibration important for a horizontal electric furnace? Ensure Precise Temperature Control for Your Materials
- What are the advantages of using a tube furnace with nitrogen control for nanoporous carbon? Enhance Your Lab Results
- What environmental parameters must high-temperature furnaces maintain for YIG thin film annealing? Expert Guide
- What is the purpose of using an industrial-grade vertical tube furnace in phosphorus recovery? High-Fidelity Simulation
- How does a high-temperature tube furnace contribute to the performance of carbon nanowire networks? Enhance Electrode Performance



















