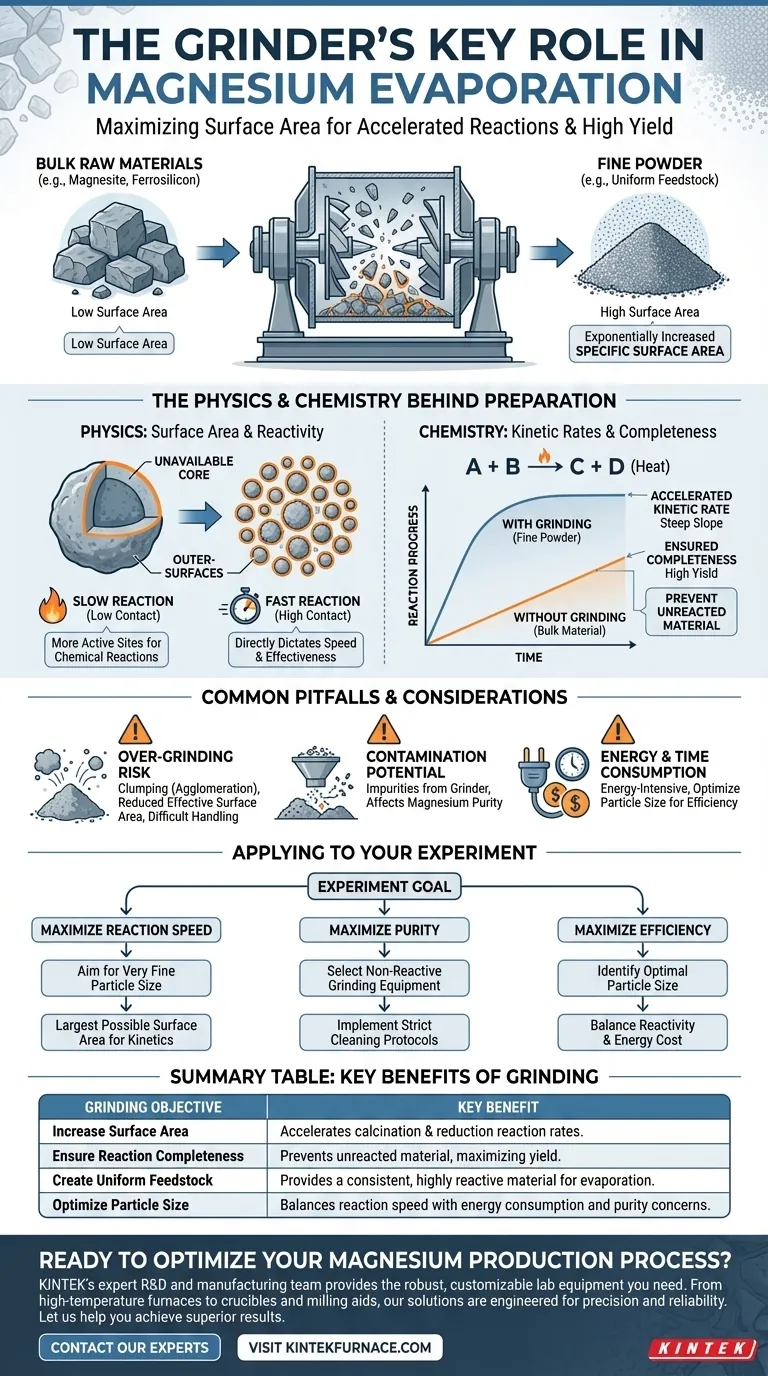
In short, the grinder's key role is to crush and refine raw materials to dramatically increase their surface area. This physical change is the critical first step that accelerates and enhances the chemical reactions required for successful magnesium production, ensuring the process is both efficient and complete.
The act of grinding is not merely about making materials smaller; it is a fundamental process that directly dictates the speed and effectiveness of the subsequent chemical reactions by maximizing the reactive surface of the raw materials.

The Physics Behind the Preparation
The success of a magnesium evaporation experiment begins long before any heating occurs. The initial physical state of the raw materials, such as magnesite and ferrosilicon alloy, sets the stage for the entire process.
The Problem with Bulk Materials
Bulk, unrefined materials have a low surface-area-to-volume ratio. This means only the outermost layer of the material is available to react, leading to slow and often incomplete chemical transformations.
The Role of Increased Surface Area
Grinding shatters these bulk materials into a fine powder. This action exponentially increases the specific surface area—the total surface area of the material exposed per unit of mass.
This newly exposed surface becomes the active site for the subsequent chemical reactions, creating a foundation for a highly efficient process.
The Chemical Impact of Grinding
By altering the physical form of the materials, the grinder directly improves two critical chemical kinetic factors: reaction rate and completeness.
Accelerating the Reaction Rate
A larger surface area allows for more contact points between the reactants at any given moment. This significantly improves the kinetic rate of the calcination and reduction reactions that produce magnesium vapor.
Essentially, the reaction can proceed much faster because more of the material is ready and available to react simultaneously.
Ensuring Reaction Completeness
With a larger reactive surface, the reactions are more likely to go to completion. This prevents unreacted material from being left behind, which increases the overall yield and efficiency of the magnesium extraction.
The ultimate outcome is the creation of a uniform and highly reactive feedstock, which is the primary goal of the preparation stage. Without this step, the experiment would be inefficient and yield poor results.
Common Pitfalls and Considerations
While crucial, the grinding process itself requires careful control to avoid introducing new problems that could compromise the experiment.
The Risk of Over-Grinding
Grinding materials too finely can sometimes be counterproductive. Extremely fine powders can be difficult to handle, may become airborne, or can lead to clumping (agglomeration), which actually reduces the effective surface area.
Potential for Contamination
The grinding equipment itself can be a source of contamination. Tiny fragments from the grinder's surfaces can mix with the raw materials, introducing impurities that may affect the purity of the final magnesium product.
Energy and Time Consumption
Grinding is an energy-intensive process. Optimizing the particle size is key—achieving a size that is fine enough for efficient reaction without wasting excessive energy or time on unnecessary milling.
Applying This to Your Experiment
The degree and method of grinding should align directly with the specific goals of your magnesium evaporation and condensation experiment.
- If your primary focus is maximizing reaction speed: You should aim for a very fine particle size to create the largest possible specific surface area, accelerating the kinetics.
- If your primary focus is maximizing purity: You must carefully select grinding equipment made from non-reactive materials and implement strict cleaning protocols to prevent contamination.
- If your primary focus is process efficiency and cost-effectiveness: You need to identify the optimal particle size that provides high reactivity without incurring excessive energy costs from over-grinding.
Ultimately, mastering the grinding stage is the first step toward achieving a controlled and successful magnesium production process.
Summary Table:
| Grinding Objective | Key Benefit for Magnesium Production |
|---|---|
| Increase Surface Area | Accelerates calcination & reduction reaction rates. |
| Ensure Reaction Completeness | Prevents unreacted material, maximizing yield. |
| Create Uniform Feedstock | Provides a consistent, highly reactive material for evaporation. |
| Optimize Particle Size | Balances reaction speed with energy consumption and purity concerns. |
Ready to Optimize Your Magnesium Production Process?
The precise preparation of raw materials is the foundation of a successful experiment. The right grinding equipment is critical for achieving the specific surface area and purity your research demands.
KINTEK's expert R&D and manufacturing team provides the robust, customizable lab equipment you need. From high-temperature furnaces for evaporation to crucibles and milling aids, our solutions are engineered for precision and reliability.
Let us help you achieve superior results. Contact our experts today to discuss how our products can be tailored to your unique magnesium evaporation and condensation experiment requirements.
Visual Guide
Related Products
- Split Multi Heating Zone Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Kiln for Pyrolysis Plant Heating
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- What types of materials can be processed in a rotary tube furnace? Discover Ideal Materials for High-Temp Processing
- How do rotary tube furnaces achieve precise temperature control? Master Uniform Heating for Dynamic Processes
- What are the common approaches to mixing in rotary furnaces? Boost Uniformity and Efficiency in Thermal Processing
- What types of materials are suitable for processing in rotary tube furnaces? Ideal for Free-Flowing Powders and Granules
- What supplementary features can enhance rotary tube furnace performance? Boost Efficiency with Precision Control



















