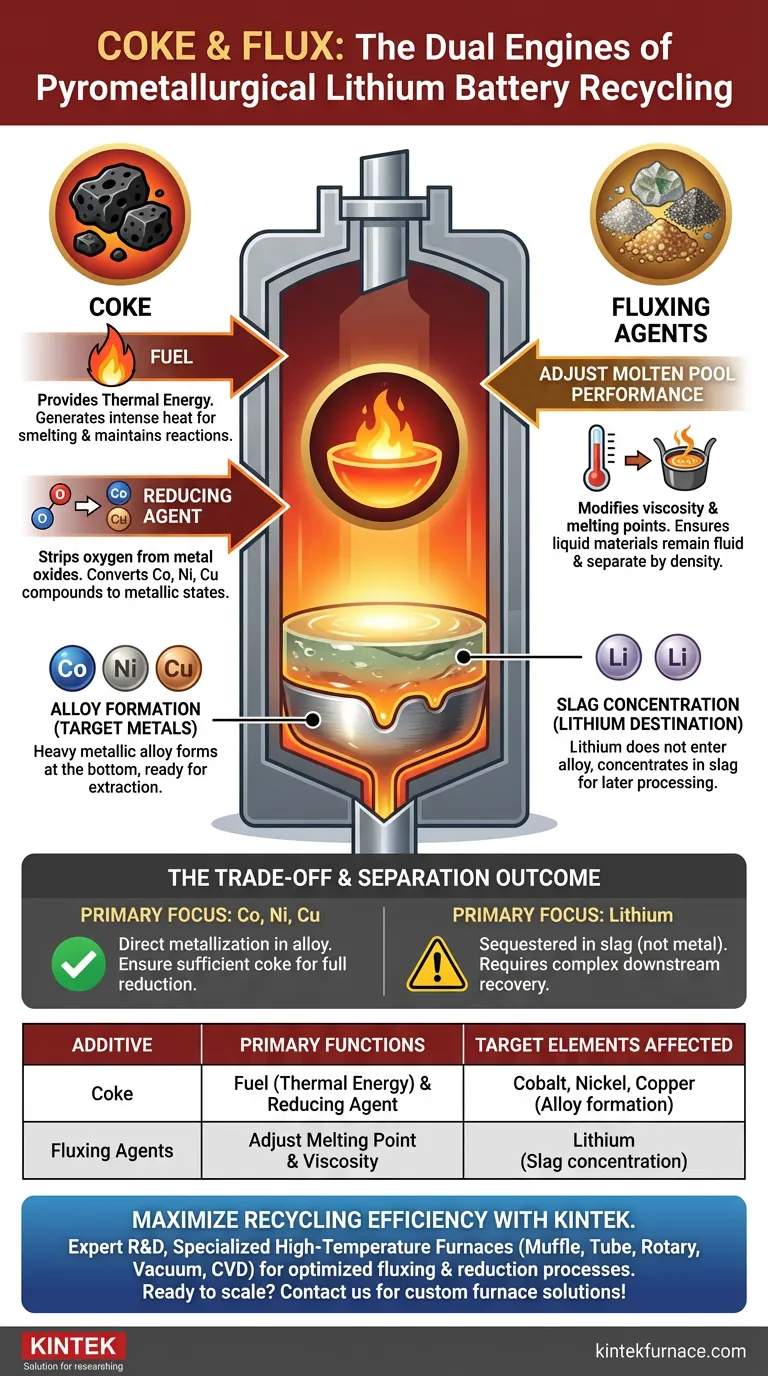
In the pyrometallurgical recycling of lithium batteries, adding coke and fluxing agents serves as the fundamental mechanism for separating valuable metals from waste. Coke performs a dual function as both a fuel to generate the necessary high temperatures and a reducing agent to chemically convert metal oxides into metallic forms. Fluxing agents are introduced to regulate the properties of the molten pool, ensuring the liquid materials separate correctly.
Core Takeaway: The interaction between these additives drives the physical separation of battery elements. Coke powers the furnace and reduces transition metals (Cobalt, Nickel, Copper) into a distinct alloy, while fluxing agents ensure the remaining materials, specifically Lithium, are concentrated within the slag for later processing.

The Dual Role of Coke
Coke is the primary driver of the reaction within the shaft furnace, serving two distinct but simultaneous purposes.
Providing Thermal Energy
First and foremost, coke acts as a fuel. The combustion of coke provides the intense heat required for high-temperature smelting.
This energy melts the battery scrap and maintains the furnace temperature necessary for chemical reactions to occur.
Acting as a Reducing Agent
Beyond simply burning, coke serves as a reducing agent. In the chemical environment of the furnace, it strips oxygen atoms from metal oxides found in the battery waste.
This reduction process is what converts compounds of cobalt, nickel, and copper back into their metallic states, allowing them to fuse together.
The Function of Fluxing Agents
While coke drives heat and reduction, fluxing agents are essential for managing the physical state of the materials inside the furnace.
Adjusting Molten Pool Performance
Fluxing agents are added to adjust the performance of the molten pool. This generally refers to modifying the viscosity and melting points of the liquid materials.
By optimizing the molten pool, the flux ensures that the materials remain fluid enough to separate physically based on density.
The Separation Outcome
The combined application of coke and flux results in the distinct "preliminary classification" of the metallic elements.
Alloy Formation (The Target Metals)
Due to the reduction provided by the coke, metals such as cobalt, nickel, and copper fuse together.
These elements form a heavy metallic alloy at the bottom of the melt, ready for extraction and further refining.
Slag Concentration (The Lithium Destination)
Unlike the transition metals, lithium does not enter the metallic alloy phase during this process.
Instead, the lithium concentrates in the slag—the non-metallic waste layer managed by the fluxing agents. This effectively separates the lithium from the high-value alloy metals.
Understanding the Trade-offs
While this process effectively recovers transition metals, it presents a specific limitation regarding lithium recovery.
Lithium is Not Recovered as Metal
The primary trade-off in this shaft furnace stage is that lithium is sequestered in the slag, not the alloy.
This means that unlike cobalt or copper, which are extracted as reduced metals, the lithium requires additional, often complex steps to be recovered from the slag material later. The process prioritizes the direct metallization of Co, Ni, and Cu over the direct recovery of Lithium.
Making the Right Choice for Your Goal
The use of coke and flux determines where specific elements end up in your recovery stream.
- If your primary focus is Cobalt, Nickel, and Copper: Ensure sufficient coke is present to act as a reducing agent, ensuring these metals fully reduce and fuse into the alloy layer.
- If your primary focus is Lithium: Recognize that in this specific pyrometallurgical setup, your lithium will be locked in the slag, requiring you to optimize your fluxing agents to ensure the slag is manageable for subsequent downstream processing.
The shaft furnace relies on these inputs to achieve the critical first step of recycling: concentrating high-value transition metals into an alloy while segregating lithium into the slag.
Summary Table:
| Additive | Primary Functions | Target Elements affected |
|---|---|---|
| Coke | Fuel (Thermal Energy) & Reducing Agent | Cobalt, Nickel, Copper (Alloy formation) |
| Fluxing Agents | Adjust Melting Point & Viscosity of Molten Pool | Lithium (Slag concentration) |
Maximize Your Battery Recycling Efficiency with KINTEK
Transitioning from lab-scale testing to industrial pyrometallurgical processing requires precision and the right thermal environment. Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, along with specialized high-temperature furnaces customizable for your unique lithium-ion battery recycling needs.
Whether you are optimizing fluxing agent performance or refining the reduction process for high-value alloys, our technical team is ready to provide the specialized equipment you need for superior material recovery.
Ready to scale your recycling results? Contact us today to explore our custom furnace solutions!
Visual Guide
References
- Vladimír Marcinov, Zita Takáčová. Overview of Recycling Techniques for Lithium-Ion Batteries. DOI: 10.15255/kui.2023.030
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant Rotating Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
People Also Ask
- Why is stepped temperature control on a laboratory hotplate necessary for drying sensor active layers? Boost Precision
- Why is charcoal used as a susceptor material during the microwave cladding of FeCoNiMnCu? Unlock efficient heating.
- How does plant metal-ion absorption influence pyrolysis? Enhance Material Synthesis with Biological Pretreatment
- Why is XPS used to analyze manganese catalysts? Master Surface Valence States for Enhanced Reactivity
- What is the temperature range of a lab furnace? Find Your Perfect Match
- Why is precise temperature control necessary for drying plum stone raw materials? Enhance Biochar Quality & Grinding
- What are the drawbacks of cold compacting and sintering? Higher Porosity and Weaker Mechanical Properties
- How do heat treatment furnaces function? Master Thermal Control and Atmosphere for Superior Material Properties



















