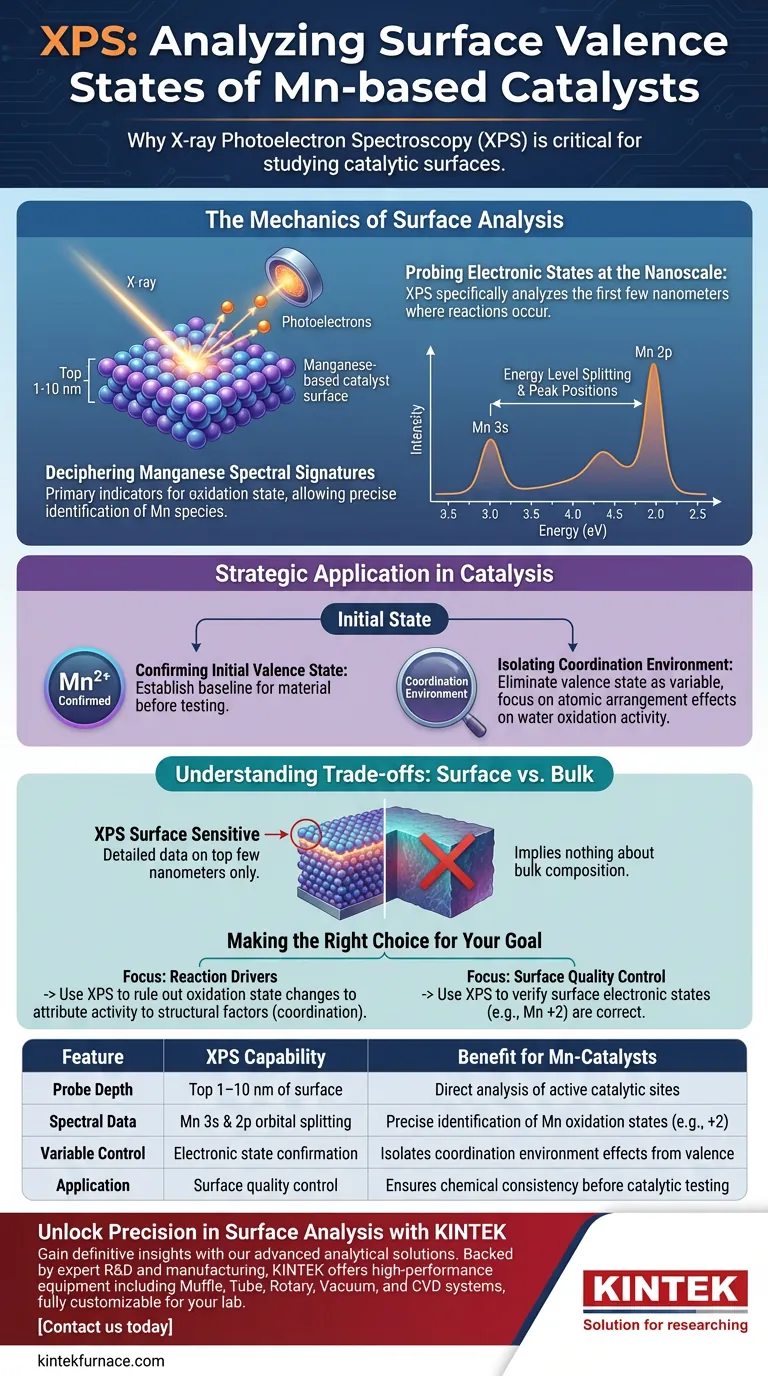
X-ray Photoelectron Spectroscopy (XPS) is utilized primarily for its ability to probe the electronic states within the first few nanometers of a material's surface. By analyzing specific spectral features, such as the energy level splitting and peak positions of Manganese (Mn) 3s and 2p orbitals, researchers can definitively confirm the initial valence state of the surface manganese. This confirmation is essential for isolating variables in complex catalytic reactions.
By confirming that the surface manganese remains in a specific valence state (such as +2), XPS eliminates oxidation state as a variable. This allows researchers to attribute differences in water oxidation activity directly to the coordination environment rather than changes in electronic charge.
The Mechanics of Surface Analysis
Probing Electronic States at the Nanoscale
XPS is distinct because it does not analyze the bulk material deep inside the sample.
It specifically probes the electronic states within the first few nanometers of the surface.
This is the region where catalytic reactions physically occur, making it the most critical area to characterize for reactivity studies.
Deciphering Manganese Spectral Signatures
To determine the exact valence state, analysts examine specific energy signatures in the spectrum.
The primary indicators are the energy level splitting and peak positions of the Mn 3s and Mn 2p orbitals.
These spectral features shift predictably depending on the oxidation state, allowing for precise identification of the manganese species present.
Strategic Application in Catalysis
Confirming the Initial Valence State
In the context of manganese phosphate research, the goal is often to establish a baseline for the material.
XPS is used to confirm that the surface manganese is specifically in a +2 valence state.
Verifying this initial state ensures that the starting material is chemically consistent before any catalytic testing begins.
Isolating the Coordination Environment
The true power of XPS in this context lies in variable isolation.
By proving the valence state is constant, researchers can effectively eliminate it as a variable affecting the reaction.
This allows the scientific focus to shift entirely to how the coordination environment—the arrangement of atoms around the manganese center—impacts water oxidation activity.
Understanding the Trade-offs
Surface vs. Bulk Composition
It is critical to remember that XPS is strictly a surface-sensitive technique.
It provides detailed data on the top few nanometers but implies nothing about the bulk of the material.
If the surface composition differs significantly from the interior, relying solely on XPS may result in an incomplete characterization of the catalyst as a whole.
Making the Right Choice for Your Goal
To determine if XPS is the correct analytical tool for your specific inquiry, consider your primary research objective:
- If your primary focus is determining reaction drivers: Use XPS to rule out oxidation state changes so you can attribute activity to structural factors like the coordination environment.
- If your primary focus is surface quality control: Use XPS to verify that the top few nanometers of your catalyst possess the specific electronic states (e.g., Mn +2) required for your reaction.
Ultimately, XPS provides the definitive electronic evidence required to disentangle valence state effects from structural geometry in catalytic performance.
Summary Table:
| Feature | XPS Capability | Benefit for Mn-Catalysts |
|---|---|---|
| Probe Depth | Top 1–10 nm of surface | Direct analysis of active catalytic sites |
| Spectral Data | Mn 3s & 2p orbital splitting | Precise identification of Mn oxidation states (e.g., +2) |
| Variable Control | Electronic state confirmation | Isolates coordination environment effects from valence |
| Application | Surface quality control | Ensures chemical consistency before catalytic testing |
Unlock Precision in Surface Analysis with KINTEK
Gain definitive insights into your catalyst's electronic structure with our advanced analytical solutions. Backed by expert R&D and manufacturing, KINTEK offers high-performance equipment including Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable for your unique lab requirements.
Whether you are refining manganese-based catalysts or developing new materials, our high-temperature systems provide the stability and control necessary for superior sample preparation. Contact us today to discuss how our specialized lab furnaces can elevate your research and manufacturing outcomes!
Visual Guide
References
- Shujiao Yang, Wei Zhang. Electrocatalytic water oxidation with manganese phosphates. DOI: 10.1038/s41467-024-45705-1
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
People Also Ask
- Why is a N2 and SF6 gas protection system required for magnesium melting? Ensure Safety and Alloy Purity
- What are the key characteristics of furnaces used in 3D printing sintering? Achieve Precision Sintering for High-Quality Parts
- What role does a laboratory facility play in establishing the mass balance for a coke oven operation? Drive Efficiency.
- What is the purpose of sintering furnaces? Transform Powders into Strong, Dense Materials
- What role does a laboratory drying oven play in the post-treatment of Cu/ZIF-8 catalysts? Ensuring Structural Integrity
- What additional benefits do vacuum chambers provide beyond environmental control? Enhance Material Purity and Process Efficiency
- What role does X-ray diffraction (XRD) play in evaluating ZIF thermal treatment? Master Material Transformation
- What is the role of a controlled hot-air circulation oven in determining the chemical composition of dried yoghurt?
