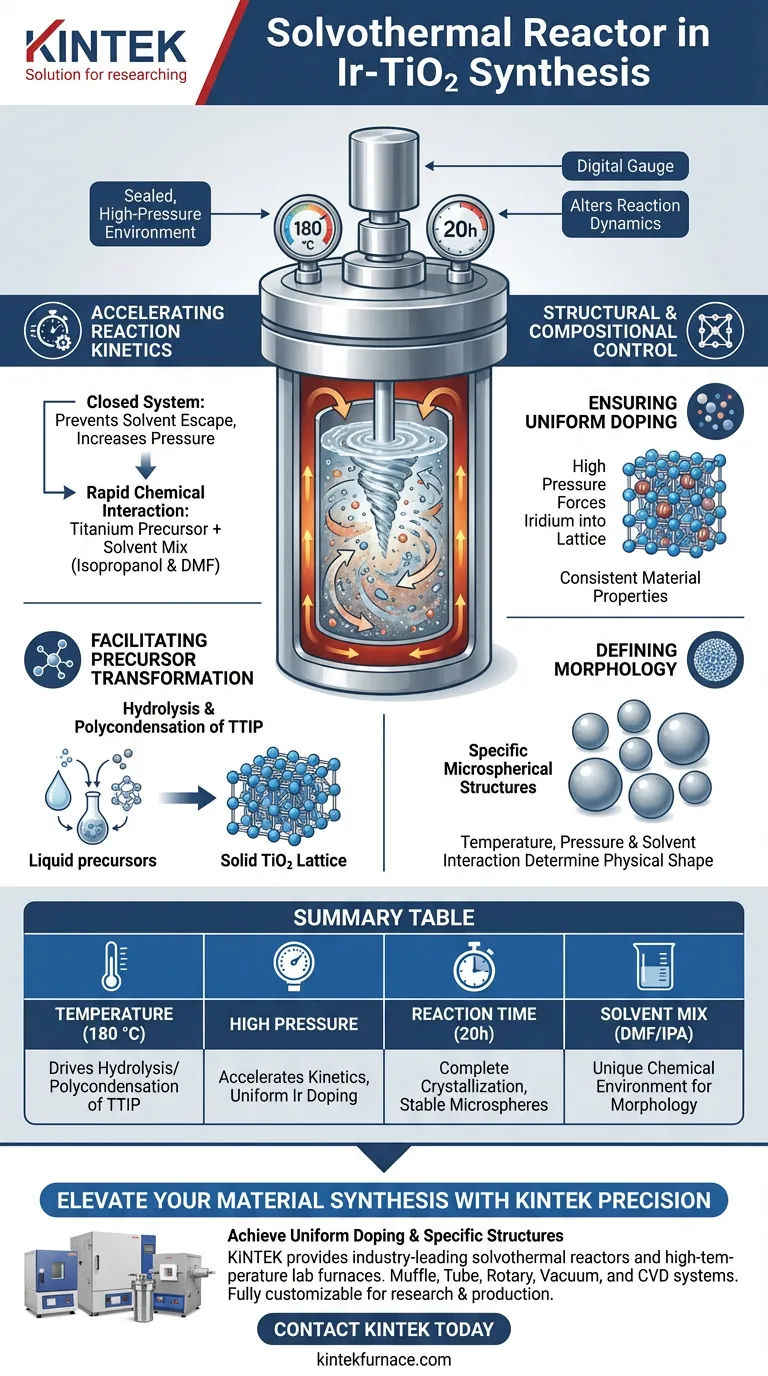
The primary function of the solvothermal reactor during the synthesis of iridium-doped titanium dioxide (Ir-TiO2) is to create a sealed, high-pressure environment that fundamentally alters reaction dynamics. By maintaining a temperature of typically 180 °C for 20 hours, the reactor facilitates the hydrolysis and polycondensation of titanium precursors, such as titanium isopropoxide (TTIP), within a specific solvent mixture.
By leveraging high pressure and temperature simultaneously, the solvothermal reactor ensures uniform iridium doping and creates specific microspherical structures that standard atmospheric heating methods cannot produce.
The Mechanism of Solvothermal Synthesis
Accelerating Reaction Kinetics
The reactor operates as a closed system, which prevents solvents from escaping and allows pressure to build significantly as temperatures rise.
This high-pressure environment accelerates the kinetics of the reaction. It forces the chemical interaction between the titanium precursor and the solvent mix of isopropanol and dimethylformamide (DMF) to occur more rapidly and completely than in open-air conditions.
Facilitating Precursor Transformation
Inside the reactor, the conditions specifically drive the hydrolysis and polycondensation of the TTIP.
This chemical transformation is the foundational step in converting the liquid precursor into the solid titanium dioxide (TiO2) lattice structure.
Structural and Compositional Control
Ensuring Uniform Doping
One of the critical roles of the solvothermal reactor is promoting the uniform doping or loading of iridium components into the TiO2 matrix.
Without the high pressure forcing the iridium into the lattice during formation, the doping would likely be uneven, leading to inconsistent material properties.
Defining Morphology
The reactor conditions are not just about chemical composition; they determine the physical shape of the final material.
The specific combination of high temperature, pressure, and solvent interaction is essential for forming specific microspherical morphologies, giving the Ir-TiO2 its unique physical structure.
Operational Constraints and Considerations
Stringent Parameter Control
The success of this synthesis relies heavily on maintaining exact conditions over a long duration.
The process requires a sustained temperature of 180 °C for 20 hours. Deviating from this time-temperature profile can disrupt the crystallization process or lead to incomplete doping.
Solvent Dependency
The reactor's efficacy is tightly coupled with the solvent system used.
The mechanism relies on the interaction between isopropanol and dimethylformamide (DMF). Using the reactor without this specific solvent blend may not generate the correct pressure or chemical environment required for the desired microspheres.
Making the Right Choice for Your Goal
To maximize the quality of your Ir-TiO2 synthesis, consider the following based on your specific objectives:
- If your primary focus is Uniform Doping: Ensure the reactor seal is perfect to maintain the high pressure required to force iridium uniformly into the TiO2 matrix.
- If your primary focus is Morphology: Strictly adhere to the mixed solvent ratio of isopropanol and DMF, as this interaction within the reactor drives the microspherical shape.
Success in this synthesis is defined by the reactor's ability to maintain a stable, high-pressure closed system for the full 20-hour duration.
Summary Table:
| Key Parameter | Function in Ir-TiO2 Synthesis |
|---|---|
| Temperature (180 °C) | Drives hydrolysis and polycondensation of titanium precursors (TTIP). |
| High Pressure | Accelerates reaction kinetics and forces uniform iridium doping into the lattice. |
| Reaction Time (20h) | Ensures complete crystallization and structural stability of microspheres. |
| Solvent Mix (DMF/IPA) | Creates the specific chemical environment for unique physical morphologies. |
Elevate Your Material Synthesis with KINTEK Precision
Achieving uniform doping and specific microspherical structures in Ir-TiO2 requires rigorous control over pressure and temperature. KINTEK provides industry-leading solvothermal reactors and high-temperature lab furnaces designed to meet these exacting standards.
Backed by expert R&D and manufacturing, we offer a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems, all fully customizable to your unique research or production needs. Don't let equipment limitations hinder your innovation—partner with KINTEK for reliable, high-performance thermal solutions.
Contact KINTEK today to discuss your custom furnace requirements!
Visual Guide
References
- Harnessing Visible Light: Unraveling the Photocatalytic Water Splitting Activity of Ir–TiO<sub>2</sub>. DOI: 10.1021/acsaem.5c01776
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Vacuum Heat Treat Sintering Furnace with Pressure for Vacuum Sintering
- Vacuum Heat Treat Sintering and Brazing Furnace
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- Split Chamber CVD Tube Furnace with Vacuum Station CVD Machine
People Also Ask
- What are the advantages of using an acid oxidation bath? Accelerate Lignin Fiber Stabilization from Hours to Minutes
- How does high-temperature filtration equipment facilitate molten salt separation? Boost Your Slag Treatment Recovery
- What challenges are associated with batch furnaces? Overcome Inefficiency and Quality Issues
- What critical environmental conditions does a high-temperature recrystallization annealing furnace provide? Maximize Steel Strength
- How does a high-precision temperature control system assist in evaluating the thermal management capabilities of phosphor materials? Pinpoint Performance for Solar Cells.
- What are the technical functions of carrier gases in VTD? Master Vapor Transport Deposition Control
- What role does a high-temperature thermal simulation system play in the dissolution of precipitates in steel?
- What is the role of industrial electric drying ovens in FDSSC titanium photoanode treatment? Enhance Solar Efficiency



















