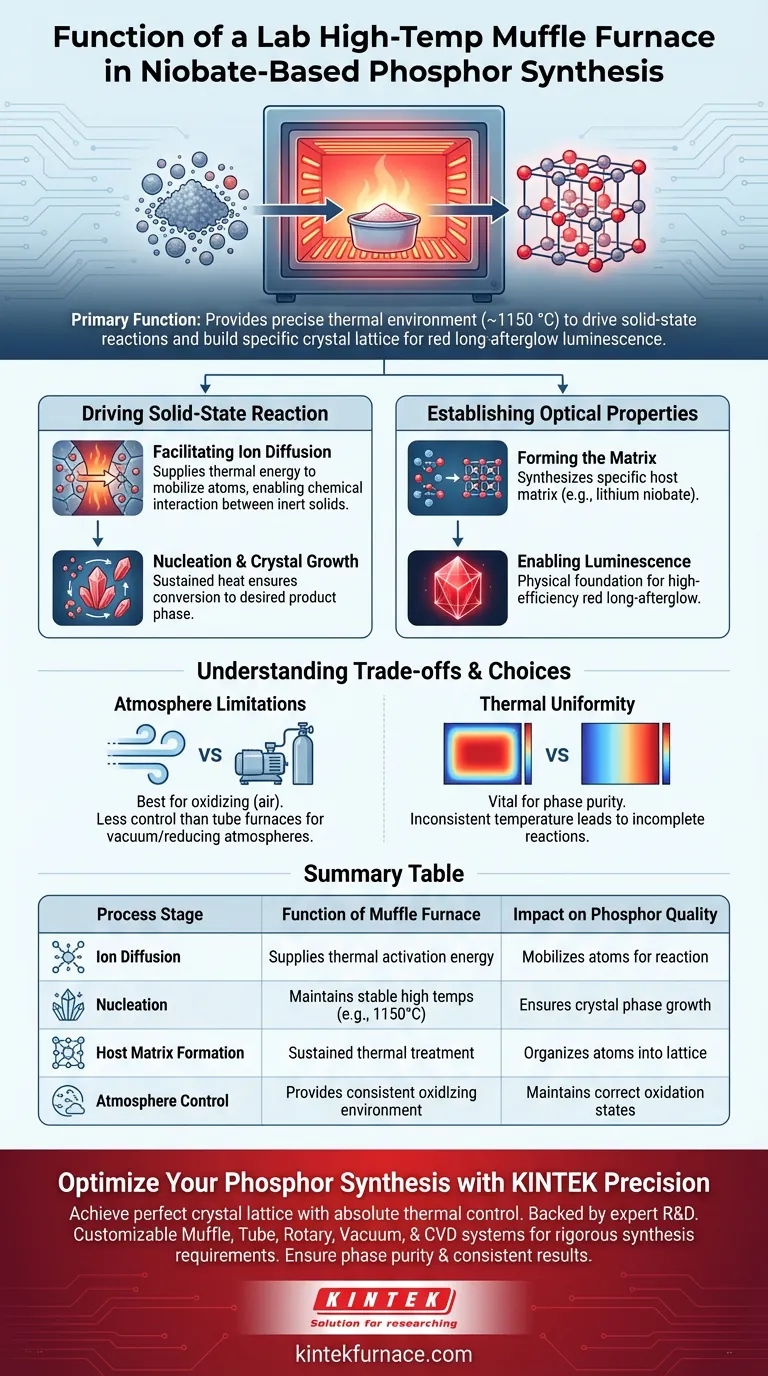
The primary function of a laboratory high-temperature muffle furnace in this context is to provide the precise thermal environment required to drive solid-state chemical reactions. Specifically for niobate-based phosphors, the furnace maintains elevated temperatures—often around 1150 °C—to transform raw powder mixtures into a unified crystalline structure.
The furnace provides the necessary thermal activation energy to facilitate ion diffusion and nucleation. This process is critical for constructing the specific crystal lattice required to achieve high-efficiency red long-afterglow luminescence.

Driving the Solid-State Reaction
Facilitating Ion Diffusion
In solid-state synthesis, the reactants are distinct solid powders that do not naturally mix.
The muffle furnace supplies the thermal energy needed to mobilize atoms within these solids.
This heat allows ions to diffuse across particle boundaries, enabling the chemical interaction between components that would otherwise remain inert.
Nucleation and Crystal Growth
Once diffusion begins, the material undergoes nucleation, where new crystal phases begin to form.
The furnace maintains the temperature for several hours to ensure these nuclei grow into stable, well-defined crystals.
This sustained heating ensures the complete conversion of raw materials into the desired product phase.
Establishing the Optical Properties
Forming the Matrix
The ultimate goal of using the furnace is to synthesize a specific host matrix, such as lithium niobate or sodium niobate.
The thermal treatment organizes the atoms into a precise lattice structure.
Enabling Luminescence
This lattice serves as the physical foundation for the material's optical properties.
Only when this specific crystal structure is perfectly formed can the material exhibit high-efficiency red long-afterglow luminescence.
Understanding the Trade-offs
Atmosphere Limitations
While high-temperature muffle furnaces are excellent for oxidizing environments (air), they have limitations regarding atmosphere control compared to tube furnaces.
If your synthesis requires a vacuum or a reducing atmosphere (like hydrogen/nitrogen mixtures), a muffle furnace may not be suitable unless specifically modified.
Standard muffle furnaces are best suited for reactions where an air environment promotes the correct oxidation state of the phosphor.
Thermal Uniformity
For high-quality phosphors, temperature consistency is vital.
Variations in temperature within the chamber can lead to "phase impurities," where parts of the sample do not fully react.
You must ensure the furnace provides a uniform high-temperature field to guarantee the entire batch achieves the correct lattice structure.
Making the Right Choice for Your Goal
To ensure the successful synthesis of niobate-based phosphors, align your equipment choice with your specific processing needs:
- If your primary focus is standard oxide synthesis: Rely on the muffle furnace for its ability to maintain a stable, oxidizing environment at 1150 °C to ensure proper lattice formation.
- If your primary focus is phase purity: Prioritize a furnace with verified temperature uniformity to prevent incomplete reactions and ensure consistent luminescence.
- If your primary focus is atmosphere control: Evaluate if the standard air environment of a muffle furnace is sufficient, or if a tube furnace is required for specialized gas flow.
Success in solid-state synthesis relies not just on reaching the right temperature, but on maintaining the precise conditions that allow the crystal lattice to mature.
Summary Table:
| Process Stage | Function of Muffle Furnace | Impact on Phosphor Quality |
|---|---|---|
| Ion Diffusion | Supplies thermal activation energy | Mobilizes atoms to cross particle boundaries for reaction |
| Nucleation | Maintains stable high temperatures (e.g., 1150°C) | Ensures new crystal phases form and grow into stable structures |
| Host Matrix Formation | Sustained thermal treatment | Organizes atoms into the lattice required for red long-afterglow |
| Atmosphere Control | Provides consistent oxidizing environment | Maintains correct oxidation states for optimal optical performance |
Optimize Your Phosphor Synthesis with KINTEK Precision
Achieving the perfect crystal lattice for high-efficiency luminescence requires absolute thermal control. Backed by expert R&D and world-class manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the rigorous demands of solid-state synthesis.
Whether you need precise atmospheric control or superior temperature uniformity for your niobate-based materials, our lab high-temp furnaces are fully customizable to your unique research needs. Ensure phase purity and consistent results every time—contact our technical specialists today to find your solution!
Visual Guide
References
- Hua Yang, Pinghui Ge. Pr3+-Doped Lithium Niobate and Sodium Niobate with Persistent Luminescence and Mechano-Luminescence Properties. DOI: 10.3390/app14072947
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What other applications do muffle furnaces have? Unlock Versatile Uses in Labs and Manufacturing
- What are the benefits of using a muffle furnace? Achieve Precise, Contamination-Free Heating for Your Lab
- Why is a constant temperature oven better than a heating plate for annealing Cs3Cu2I5:Tb films? Expert Comparison
- What are some advancements in modern muffle furnace technology? Boost Precision and Efficiency in Your Lab
- What are some common uses of muffle furnaces in material testing? Unlock Precise Heat Treatment for Accurate Results
- What is the process logic of a muffle furnace for tungsten oxynitride thin films? Optimize Your Thermal Cycle
- How are muffle furnaces used in electronics manufacturing? Essential for Precision Thermal Processing
- How do sample characteristics affect muffle furnace selection? Ensure Accurate and Safe High-Temperature Processing



















