A laboratory gas-phase catalytic reaction system functions as a high-fidelity verification tool designed to assess the actual performance of carbon-metal nanocomposites in a controlled environment. By utilizing a quartz tube reactor and precise thermal controls, the system exposes the material to reactant gases to determine its efficacy in driving chemical transformations, such as decomposing ammonia into hydrogen.
The core value of this system is its ability to simulate an industrial electrified chemical synthesis environment. It moves beyond theoretical material properties to provide real-time, empirical data on how a composite behaves under operating conditions.

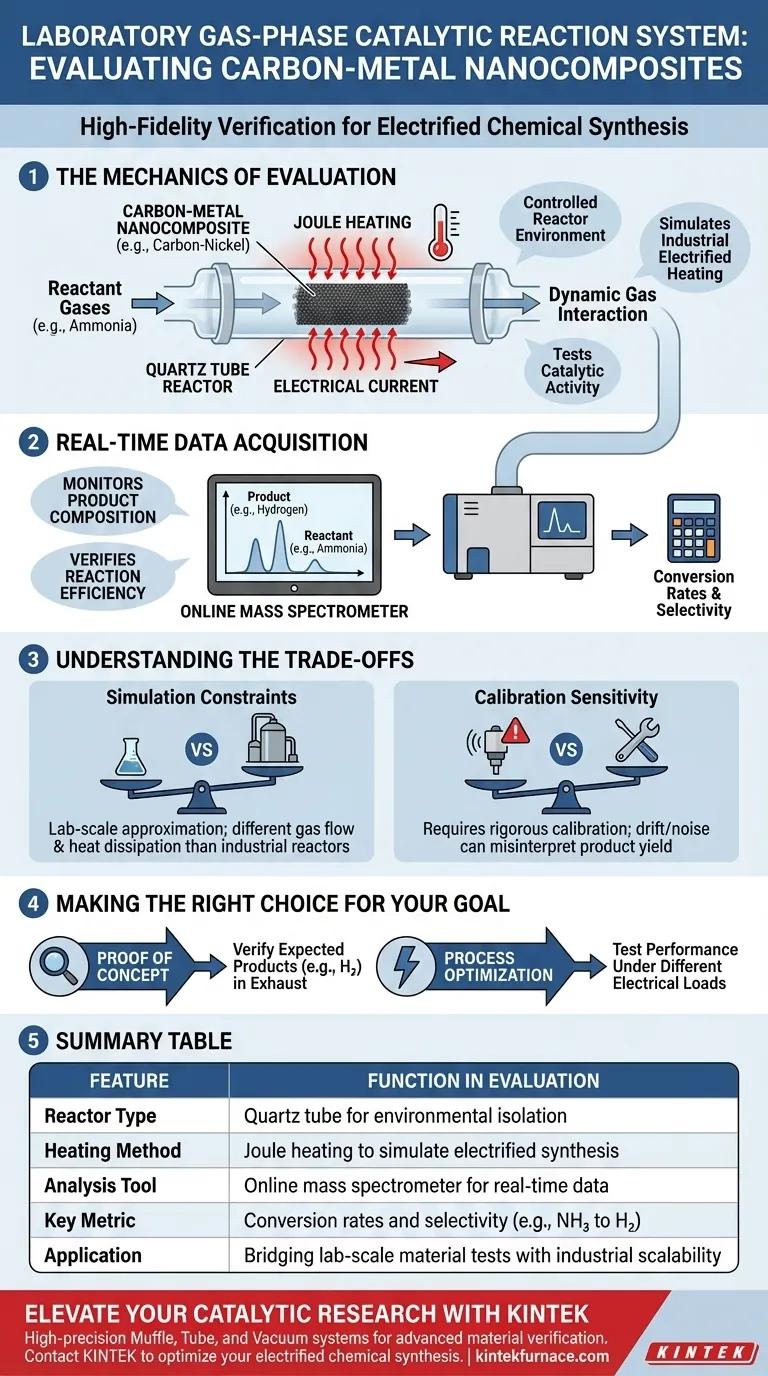
The Mechanics of Evaluation
Creating a Controlled Reactor Environment
The foundation of the system is a quartz tube reactor. This component isolates the carbon-metal nanocomposite (such as carbon-nickel) from the outside environment.
This isolation ensures that any chemical changes observed are solely the result of the interaction between the catalyst and the reactant gases.
Simulating Electrified Heating
To mimic modern industrial processes, the system employs Joule heating. Instead of heating the reactor from the outside, electrical current is passed through the composite material itself.
This generates heat directly within the catalyst. High-precision thermal monitoring is used simultaneously to ensure the material reaches and maintains the exact temperatures required for reaction.
Dynamic Gas Interaction
Reactant gases, specifically ammonia in this context, are passed over the heated composite.
This flow tests the material's catalytic activity—its ability to break chemical bonds and facilitate reactions under thermal stress.
Real-Time Data Acquisition
Monitoring Product Composition
A critical feature of this system is its integration with an online mass spectrometer.
This device continuously samples the gas exiting the reactor. It provides immediate feedback on what chemical species are present.
Verifying Reaction Efficiency
By analyzing the output gas, researchers can confirm if the target product (e.g., hydrogen) is being produced.
This allows for the quantification of conversion rates and selectivity, determining if the nanocomposite is a viable candidate for large-scale application.
Understanding the Trade-offs
Simulation Constraints
While this system effectively simulates electrified chemical synthesis, it remains a laboratory-scale approximation.
Factors such as gas flow dynamics and heat dissipation in a small quartz tube may differ significantly from those in a massive industrial reactor.
Calibration Sensitivity
The reliance on online mass spectrometry introduces a requirement for rigorous calibration.
Drift in the sensor or background noise can lead to misinterpretation of the product yield, requiring constant vigilance during experimentation.
Making the Right Choice for Your Goal
To get the most out of a gas-phase catalytic reaction system, align your testing protocol with your specific objectives:
- If your primary focus is proof of concept: Use the online mass spectrometer to rigorously verify that the specific expected products (like hydrogen) are appearing in the exhaust stream.
- If your primary focus is process optimization: Leverage the Joule heating capability to test how the material performs under different electrical loads, simulating various industrial energy inputs.
This system effectively bridges the gap between material synthesis and viable industrial application.
Summary Table:
| Feature | Function in Evaluation |
|---|---|
| Reactor Type | Quartz tube reactor for environmental isolation |
| Heating Method | Joule heating to simulate electrified industrial synthesis |
| Analysis Tool | Online mass spectrometer for real-time gas composition |
| Key Metric | Conversion rates and selectivity (e.g., ammonia to hydrogen) |
| Application | Bridging lab-scale material tests with industrial scalability |
Elevate Your Catalytic Research with KINTEK
Transition from theoretical material design to proven industrial performance. KINTEK provides high-precision Muffle, Tube, and Vacuum systems specifically engineered for advanced chemical synthesis and material verification. Backed by expert R&D and manufacturing, our systems—including customizable CVD and high-temperature furnaces—are designed to meet the rigorous demands of carbon-metal nanocomposite testing.
Ready to optimize your electrified chemical synthesis? Contact KINTEK today to discuss how our customizable laboratory solutions can deliver the empirical data you need for your next breakthrough.
Visual Guide
References
- Paul N. Smith, Zhe Qiang. Transformative 3D Printing of Carbon‐metal Nanocomposites as Catalytic Joule Heaters for Enhanced Ammonia Decomposition. DOI: 10.1002/advs.202413149
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- RF PECVD System Radio Frequency Plasma Enhanced Chemical Vapor Deposition
- MPCVD Machine System Reactor Bell-jar Resonator for Lab and Diamond Growth
- Cylindrical Resonator MPCVD Machine System for Lab Diamond Growth
- Custom Made Versatile CVD Tube Furnace Chemical Vapor Deposition CVD Equipment Machine
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the necessity of baking electrode sheets in a vacuum oven? Ensure Battery Stability and Peak Performance
- What role does a nitrogen protection device play in copper-based halide thin films? Optimize Your Lab Annealing Process
- Why are precision stirring and drying equipment necessary for photocatalytic materials? Master Microstructure Control
- How does a high-precision temperature control system assist in evaluating the thermal management capabilities of phosphor materials? Pinpoint Performance for Solar Cells.
- Why is the water quenching process necessary for high-entropy alloys? Master Phase Purity and Microstructural Integrity
- What is the primary role of the Thermal Oxidation (TO) process in Ti-6Al-4V ELI alloy? Enhancing Hardness and Wear
- What is the objective of placing TC4 titanium alloy parts on asbestos pads? Control Stress and Thermal Shock
- How does a box heater work? A Guide to Efficient Whole-Room Heating



















