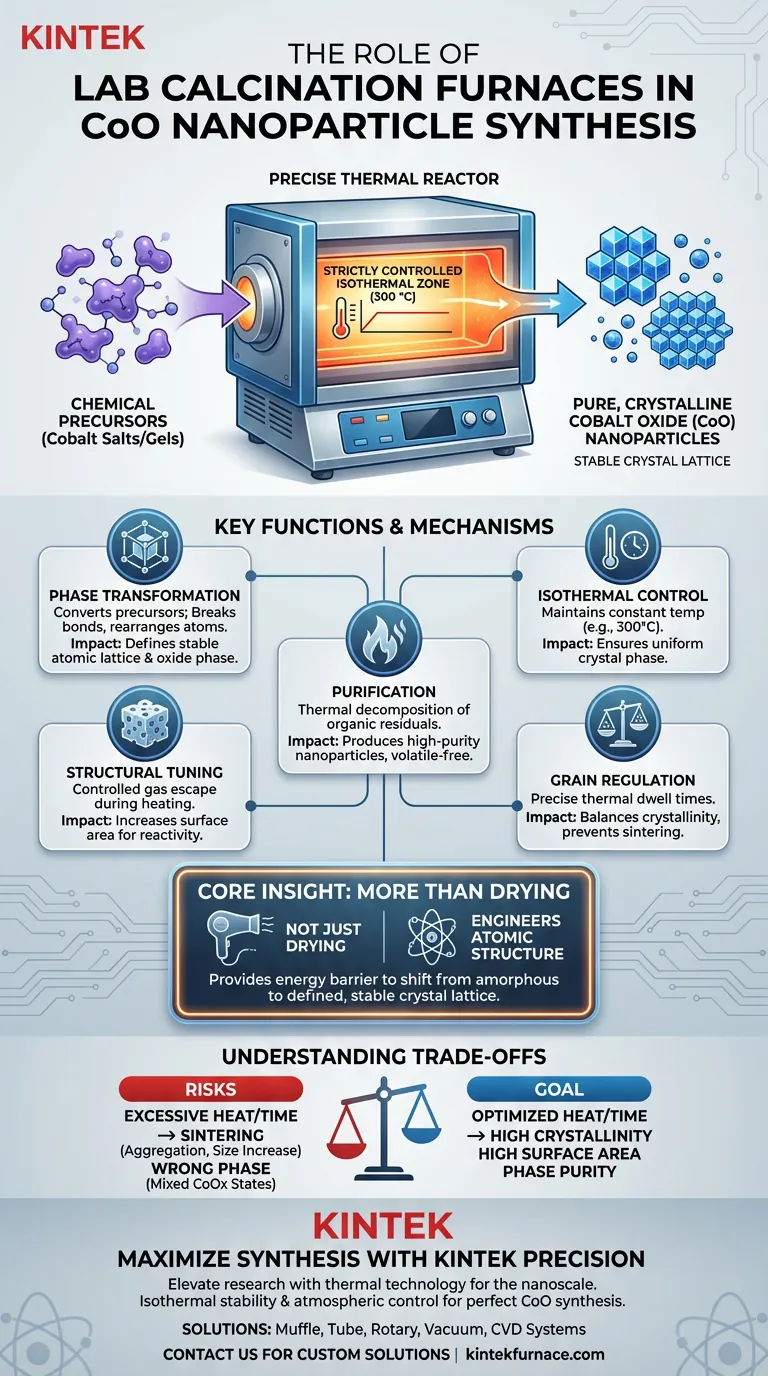
A lab calcination furnace serves as the precise thermal reactor required to convert chemical precursors into stable cobalt oxide (CoO) nanoparticles. By maintaining strictly controlled thermodynamic conditions—specifically isothermal heating around 300 °C—the furnace drives the complete transformation of cobalt salt precipitates into a pure, crystalline oxide phase while simultaneously eliminating volatile residuals.
Core Insight: The furnace does not simply dry the material; it engineers the material's atomic structure. It provides the energy barrier needed to shift the substance from an amorphous or precursor state into a defined, stable crystal lattice, ensuring the final nanoparticles possess the required purity and physical properties.

The Mechanism of Phase Transformation
Converting Precursors to Crystals
The primary function of the furnace is to facilitate a solid-state reaction. Chemical precursors, often cobalt salts or gels, are thermodynamically unstable compared to the desired oxide. The furnace provides the thermal energy required to break the chemical bonds of the precursor and rearrange the atoms into the stable crystalline structure of Cobalt Oxide (CoO).
Precise Thermodynamic Control
Achieving high-quality nanoparticles requires more than just high heat; it requires isothermal stability. The furnace creates a uniform environment where the temperature is held constant (e.g., at 300 °C). This ensures that every particle in the batch undergoes the same thermal history, resulting in a uniform crystal phase rather than a mixture of under-reacted and over-reacted material.
Purification and Structural Enhancement
Removal of Volatile Impurities
During synthesis, precursors are often mixed with solvents, surfactants, or organic stabilizers. The calcination furnace acts as a purification chamber. Through continuous high-temperature oxidation, strictly controlled heating burns off these residual organic components and volatile impurities. This is critical for obtaining high-purity nanoparticles free from contaminants that could hinder performance.
Surface Area Development
As residual gases and volatile components escape the material during heating, they often leave behind voids. This process can engineer a porous, sponge-like structure within the nanoparticles. This increase in specific surface area is vital for applications requiring high reactivity, such as catalysis, where more exposed surface area equates to better performance.
Understanding the Trade-offs
The Balance of Crystallinity vs. Aggregation
While higher temperatures or longer dwell times improve crystallinity and remove more impurities, they also carry risks. Excessive heat can cause the nanoparticles to sinter (fuse together), leading to a significant increase in grain size and a loss of the desired nanoscale surface area.
Phase Stability Risks
Cobalt can exist in multiple oxide states (e.g., CoO, Co3O4). The furnace's atmosphere and temperature precision are the only safeguards against forming the wrong phase. Inaccurate temperature control or uneven heating can lead to mixed-phase materials, compromising the electronic or magnetic properties of the final product.
Making the Right Choice for Your Goal
To optimize your cobalt oxide synthesis, align your furnace parameters with your specific performance metrics:
- If your primary focus is high purity and perfect crystallinity: Prioritize a furnace with exceptional isothermal stability to ensure complete organic decomposition and uniform crystal lattice arrangement.
- If your primary focus is catalytic activity: Focus on the ramp rate and gas escape dynamics; a controlled release of volatiles creates the porous structure necessary for maximum surface area.
The lab calcination furnace is the defining tool that bridges the gap between raw chemical potential and functional, high-performance nanomaterials.
Summary Table:
| Function | Description | Impact on CoO Nanoparticles |
|---|---|---|
| Phase Transformation | Converts cobalt precursors into crystalline structures | Defines stable atomic lattice and oxide phase |
| Isothermal Control | Maintains constant temperature (e.g., 300°C) | Ensures uniform crystal phase across the batch |
| Purification | Thermal decomposition of organic residuals | Produces high-purity nanoparticles free of volatiles |
| Structural Tuning | Controlled gas escape during heating | Increases surface area for catalytic reactivity |
| Grain Regulation | Precise thermal dwell times | Balances crystallinity with prevention of sintering |
Maximize Your Material Synthesis with KINTEK Precision
Elevate your research and production with thermal technology designed for the nanoscale. Whether you are engineering catalysts or electronic materials, KINTEK provides the isothermal stability and atmospheric control necessary for perfect cobalt oxide synthesis.
Backed by expert R&D and manufacturing, KINTEK offers a comprehensive range of Muffle, Tube, Rotary, Vacuum, and CVD systems. All our lab high-temp furnaces are fully customizable to meet your unique thermodynamic needs.
Ready to achieve superior crystallinity and purity? Contact us today to find your custom solution!
Visual Guide
References
- Kyfti Yolanda Siburian, Agung Nugroho. Effect of CoO loading on electrochemical properties of activated carbon from sugarcane bagasse. DOI: 10.5599/jese.2439
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
People Also Ask
- Why is a water-cooling spray system implemented in annealing? Maximize Production Throughput & Material Quality
- How is helium utilized in atmosphere furnaces? Unlock Purity and Rapid Cooling for Superior Results
- Why is a controlled atmosphere necessary in industrial debinding furnaces? Master the Switch from Nitrogen to Air
- How is the sealing performance of an experimental box type atmosphere furnace enhanced? Boost Purity with Advanced Sealing Systems
- How does a box type atmosphere furnace achieve precise atmosphere control? Discover Key Systems for Reliable Heat Treatment
- What types of gases are used in controlled atmosphere furnaces? Optimize Material Protection and Transformation
- What are the key benefits of using argon in furnaces? Ensure Maximum Purity and Performance
- How does the atmosphere system in a box-type atmosphere furnace work? Master Precise Chemical Control for Material Processing



















