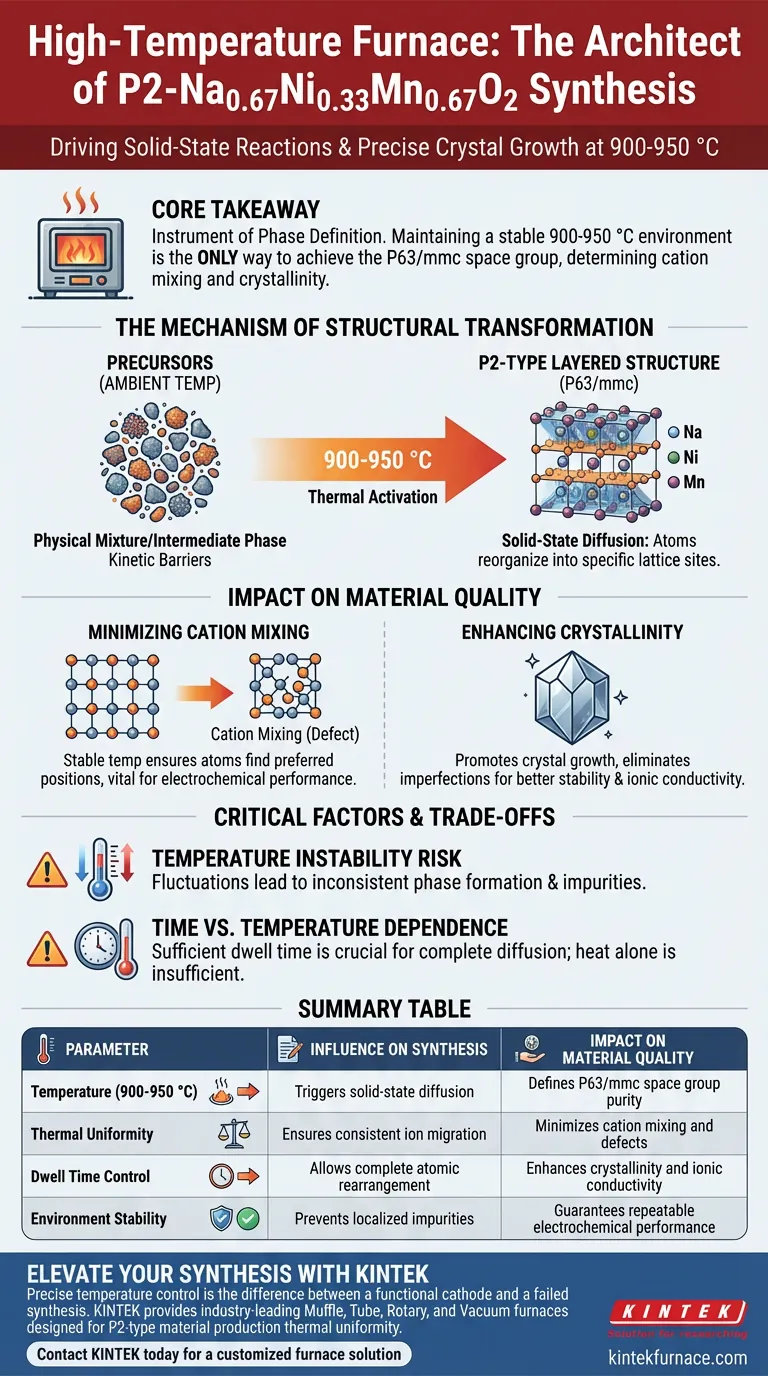
The high-temperature environment provided by the furnace acts as the essential driving force for solid-state reactions and precise crystal growth. specifically within the 900-950 °C range, this thermal energy triggers a structural reorganization of precursors, allowing sodium, nickel, and manganese ions to migrate into their correct lattice sites to form the P2-type layered structure.
Core Takeaway The muffle or box furnace is not merely a heating device; it is the instrument of phase definition. Maintaining a stable 900-950 °C environment is the only way to achieve the P63/mmc space group characteristics required for this material, directly determining the reduction of cation mixing and the degree of crystallinity in the final product.

The Mechanism of Structural Transformation
Driving Solid-State Diffusion
At ambient or lower temperatures, the precursor materials remain a physical mixture or an intermediate phase. The 900-950 °C range provides the necessary thermal activation energy to overcome kinetic barriers.
This energy enables the solid-state diffusion of atoms, allowing them to move through the solid material to rearrange themselves. This is the fundamental mechanism that transforms the raw precursors into a unified chemical compound.
Forming the P63/mmc Layered Structure
The primary goal of this synthesis stage is to achieve a specific crystallographic arrangement known as the P2-type structure (space group P63/mmc).
During this heating phase, sodium, nickel, and manganese elements are forced into specific lattice sites. The furnace ensures that these elements order themselves into distinct layers, which is the defining characteristic of P2-type cathode materials.
The Impact on Material Quality
Minimizing Cation Mixing
One of the most critical roles of this thermal treatment is the reduction of cation mixing. This phenomenon occurs when transition metal ions and alkali metal ions (like sodium) incorrectly swap places in the crystal lattice.
A stable temperature field within the 900-950 °C range ensures that atoms have sufficient energy and time to find their thermodynamically preferred positions. This distinct layering is vital for the electrochemical performance of the final battery material.
Enhancing Crystallinity
The duration and stability of the heat treatment directly influence the crystallinity of the material. High crystallinity implies a well-ordered atomic structure with fewer defects.
By maintaining the target temperature, the furnace promotes crystal growth and the elimination of structural imperfections. High crystallinity is generally correlated with better stability and ionic conductivity in the final application.
Understanding the Trade-offs
The Risk of Temperature Instability
While the target is 900-950 °C, the stability of that temperature field is just as important as the value itself. Fluctuations in the furnace can lead to inconsistent phase formation.
If the temperature drops below the effective range locally, the solid-state reaction may remain incomplete, leading to impurities. Conversely, excessive heat or hotspots could alter the stoichiometry or morphology in unintended ways.
Time vs. Temperature Dependence
The primary reference highlights that sufficient reaction time is crucial alongside temperature. This is a coupled variable; simply reaching 950 °C is insufficient if the dwell time is too short to allow for complete diffusion.
You must view the furnace process as a function of both heat and time. Cutting the heating duration short to save energy will likely result in a material with high cation mixing, regardless of whether the correct peak temperature was reached.
Making the Right Choice for Your Goal
To ensure the successful synthesis of P2-Na0.67Ni0.33Mn0.67O2, you must prioritize the precision of your thermal equipment.
- If your primary focus is Phase Purity: Ensure your furnace can hold a tight tolerance within the 900-950 °C window to guarantee the formation of the P63/mmc space group.
- If your primary focus is Electrochemical Performance: Prioritize a furnace with excellent thermal uniformity to minimize cation mixing, which directly degrades battery capacity and cycling stability.
The furnace is the architect of your material's atomic structure; precise control here is the difference between a functional cathode and a failed synthesis.
Summary Table:
| Parameter | Influence on Synthesis | Impact on Material Quality |
|---|---|---|
| Temperature (900-950 °C) | Triggers solid-state diffusion | Defines P63/mmc space group purity |
| Thermal Uniformity | Ensures consistent ion migration | Minimizes cation mixing and defects |
| Dwell Time Control | Allows complete atomic rearrangement | Enhances crystallinity and ionic conductivity |
| Environment Stability | Prevents localized impurities | Guarantees repeatable electrochemical performance |
Elevate Your Material Synthesis with KINTEK
Precise temperature control is the difference between a functional cathode and a failed synthesis. KINTEK provides industry-leading Muffle, Tube, Rotary, and Vacuum furnaces specifically designed to meet the rigorous thermal uniformity requirements of P2-type material production.
Backed by expert R&D and manufacturing, our systems are fully customizable to your unique research or production needs. Ensure your materials achieve peak crystallinity and minimal cation mixing with our advanced high-temp solutions.
Contact KINTEK today for a customized furnace solution
Visual Guide
References
- Yongchun Li, Philipp Adelhelm. Competing Mechanisms Determine Oxygen Redox in Doped Ni–Mn Based Layered Oxides for Na‐Ion Batteries. DOI: 10.1002/adma.202309842
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace with Bottom Lifting
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What temperature range do Box Furnaces operate at? From 1100°F to 3300°F for Precision Heat Treatment
- What are the core functions of a muffle furnace in the annealing process of SnO2 films? Optimize Your TCO Performance
- What is the function of a Muffle Furnace in the LSS process for MXene synthesis? Achieve Low-Temp Precision
- What role does a laboratory muffle furnace play in cotton waste biochar? Precision Pyrolysis for Carbonization
- What are the key components of a muffle furnace as shown in its diagram? Discover Its Core Architecture
- What are the common applications of the box furnace? Unlock Precise Heat Treatment for Your Lab
- What is the significance of box type electric furnaces in metal melting? Precision Control for Small-Scale Metallurgy
- What is the function of a muffle furnace during catalyst calcination? Master Biomass-to-Catalyst Transformation



















