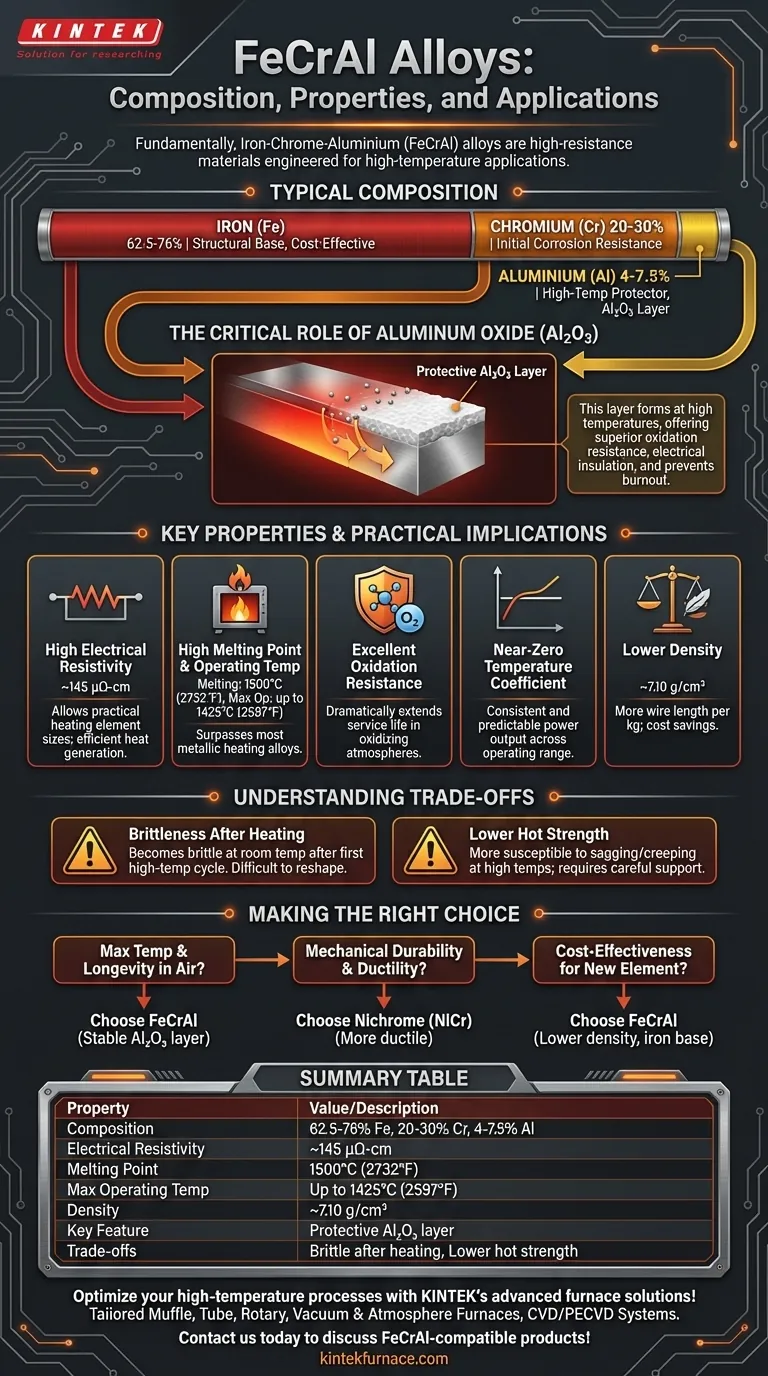
Fundamentally, Iron-Chrome-Aluminium (FeCrAl) alloys are a family of high-resistance materials specifically engineered for high-temperature applications. Their typical composition is 62.5-76% Iron (Fe), 20-30% Chromium (Cr), and 4-7.5% Aluminium (Al). This specific combination gives them their signature properties: extremely high electrical resistivity, a high melting point, and world-class resistance to oxidation at elevated temperatures.
The defining characteristic of FeCrAl alloys is not just their high resistance, but the formation of a stable, protective aluminum oxide layer at high temperatures. This layer is the key to their superior performance and longevity in harsh electric heating environments.

The Role of Each Element
To understand FeCrAl, you must understand how its three primary components work together. Each element plays a distinct and critical role in the alloy's overall performance.
Iron (Fe): The Structural Base
Iron serves as the primary matrix of the alloy. As the most abundant element, it provides the structural foundation and is a key reason for FeCrAl's cost-effectiveness compared to nickel-based alternatives.
Chromium (Cr): Initial Corrosion Resistance
Chromium is essential for providing general corrosion and oxidation resistance, particularly at lower temperatures. It readily forms a passive chromium oxide layer that protects the alloy from its environment.
Aluminum (Al): The High-Temperature Protector
Aluminum is the most important element for high-temperature performance. When heated, the aluminum migrates to the surface and oxidizes, forming a thin, dense, and highly adherent layer of aluminum oxide (Al₂O₃), also known as alumina.
This alumina layer is chemically stable, electrically insulating, and has a very high melting point. It is this self-repairing protective skin that prevents the underlying alloy from burning out in the presence of oxygen at extreme temperatures.
Key Properties and Their Practical Implications
The composition of FeCrAl directly translates to a set of properties that make it ideal for specific engineering challenges, primarily in the field of electrical heating.
High Electrical Resistivity
FeCrAl exhibits a very high resistivity of around 145 μΩ-cm. For a heating element, this is crucial. It allows a component of a practical size and length to generate significant heat (governed by the formula P = I²R) without requiring excessively high current.
High Melting Point and Operating Temperature
With a melting point of 1500°C (2732°F), the alloy can operate at very high temperatures. More importantly, the stable alumina layer allows for a maximum continuous operating temperature of up to 1425°C (2597°F) for some grades, surpassing most other metallic heating alloys.
Excellent Oxidation Resistance
As mentioned, this is FeCrAl's standout feature. The Al₂O₃ layer provides exceptional protection in oxidizing atmospheres (like open air), dramatically extending the service life of heating elements in furnaces, kilns, and appliances.
Near-Zero Temperature Coefficient
This property means the alloy's resistance does not change significantly as its temperature increases. This stability ensures predictable and consistent power output from the heating element across its entire operating range.
Lower Density
FeCrAl has a specific gravity of around 7.10 g/cm³. This is notably less dense than competing nickel-chromium (Nichrome) alloys. For designers, this means you get more wire length per kilogram, which can result in a significant material cost savings for a given project.
Understanding the Trade-offs
No material is perfect. Acknowledging FeCrAl's limitations is critical for proper application and design.
Brittleness After Heating
After its first high-temperature heat cycle, FeCrAl undergoes a grain growth that makes it brittle at room temperature. While it remains functional at high temperatures, it cannot be easily bent, reshaped, or serviced once cooled without risk of fracture.
Lower Hot Strength
Compared to nickel-based alloys like Nichrome, FeCrAl can have lower mechanical strength at its highest operating temperatures. This makes it more susceptible to sagging or "creeping" under its own weight and requires careful mechanical support in furnace designs.
Making the Right Choice for Your Application
Your choice of material depends entirely on the specific demands of your project.
- If your primary focus is maximum operating temperature and longevity in air: FeCrAl is often the superior choice due to its highly stable aluminum oxide protective layer.
- If your primary focus is mechanical durability and ductility after use: Nichrome (NiCr) alloys may be a better option, as they remain more ductile and are less prone to creep at high temperatures.
- If your primary focus is cost-effectiveness for a new heating element: FeCrAl's lower density and iron base can provide a significant cost advantage over nickel-based alternatives.
Ultimately, understanding the role of aluminum in creating its protective oxide layer is the key to effectively leveraging FeCrAl's unique capabilities.
Summary Table:
| Property | Value / Description |
|---|---|
| Composition | 62.5-76% Fe, 20-30% Cr, 4-7.5% Al |
| Electrical Resistivity | ~145 μΩ-cm |
| Melting Point | 1500°C (2732°F) |
| Max Operating Temperature | Up to 1425°C (2597°F) |
| Density | ~7.10 g/cm³ |
| Key Feature | Forms protective Al₂O₃ layer for oxidation resistance |
| Trade-offs | Brittle after heating, lower hot strength vs. NiCr alloys |
Optimize your high-temperature processes with KINTEK's advanced furnace solutions! Leveraging exceptional R&D and in-house manufacturing, we provide diverse laboratories with tailored high-temperature furnace systems, including Muffle, Tube, Rotary, Vacuum & Atmosphere Furnaces, and CVD/PECVD Systems. Our strong deep customization capability ensures we precisely meet your unique experimental needs, enhancing efficiency and durability. Contact us today to discuss how our FeCrAl-compatible products can elevate your research and industrial applications!
Visual Guide
Related Products
- Molybdenum Disilicide MoSi2 Thermal Heating Elements for Electric Furnace
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- Laboratory Muffle Oven Furnace with Bottom Lifting
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- Why are SiC heating elements considered environmentally friendly? Discover Their Eco-Efficiency & Lifespan Insights
- What is the purpose of wrapping heating tapes around AP-SCVD gas lines? Prevent Condensation for Perfect Film Quality
- What materials are typically used in the construction of high temperature heating elements? Discover the Best Options for Your Needs
- Why do heating coils in high-temp electric furnaces require power regulators? Ensure Precision in Metal Processing
- How do silicon carbide heating elements reduce operating costs? Achieve Long-Term Savings and Efficiency
- Why are high-performance microwave-absorbing materials required in microwave sintering? Solve the 'Cold Start' Challenge
- How does a K-type thermocouple ensure process reliability in lead-bismuth alloy separation? Master Thermal Precision
- What are the typical applications of molybdenum heating elements? Powering High-Temp Furnace Processes



















