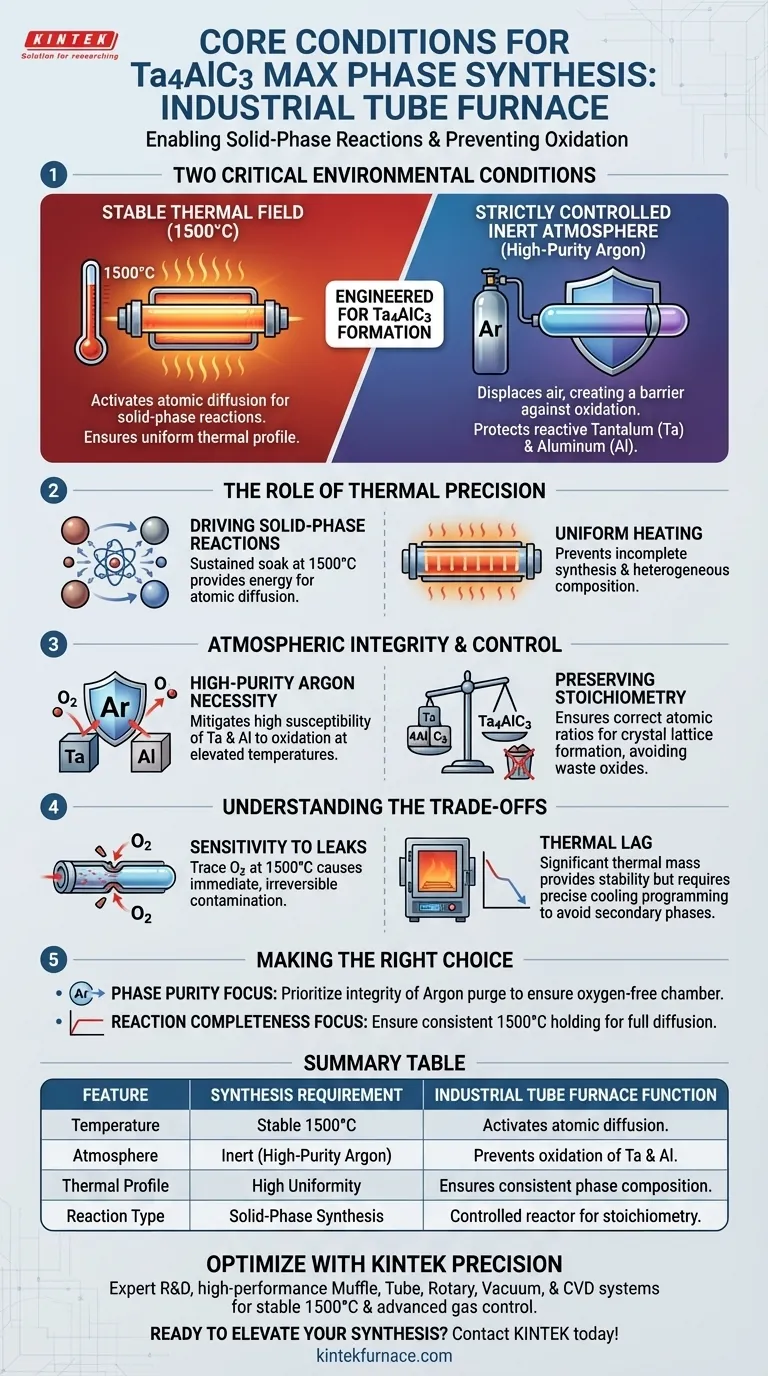
For the synthesis of Ta4AlC3 MAX phases, an industrial high-temperature tube furnace provides two critical environmental conditions: a stable thermal field capable of reaching 1500°C and a strictly controlled inert atmosphere, typically utilizing high-purity argon. This specific combination is engineered to facilitate solid-phase reactions among raw powder precursors. Crucially, this environment isolates the material to prevent the oxidation of reactive elements—specifically tantalum and aluminum—which would otherwise degrade rapidly at these elevated temperatures.
The tube furnace functions not just as a heater, but as an isolated chemical reactor that stabilizes the thermodynamics required to form Ta4AlC3 while actively suppressing the formation of unwanted oxides.

The Role of Thermal Precision
Driving Solid-Phase Reactions
The synthesis of Ta4AlC3 is not a simple melting process; it relies on solid-phase reactions.
To achieve this, the furnace must provide a sustained soak temperature of 1500°C. This high energy input is necessary to activate the diffusion of atoms between the solid particles of the raw precursors.
Uniform Heating
Beyond peak temperature, the tube furnace ensures a uniform thermal profile.
This prevents localized hot or cold spots that could lead to incomplete synthesis or heterogeneous phase composition within the sample batch.
Atmospheric Integrity and Control
The Necessity of High-Purity Argon
At 1500°C, the metallic components of the MAX phase precursor—specifically tantalum and aluminum—are highly susceptible to reacting with oxygen.
The tube furnace mitigates this by maintaining a protective atmosphere of high-purity argon. This inert gas displaces air within the tube, creating a barrier that prohibits oxidative degradation.
Preserving Stoichiometry
The quality of a MAX phase material depends on maintaining exact atomic ratios.
By effectively preventing oxidation, the furnace ensures that the aluminum and tantalum remain available to form the Ta4AlC3 crystal lattice rather than being consumed as waste oxides (slag).
Understanding the Trade-offs
Sensitivity to Leaks
While the tube furnace is excellent at atmospheric control, the system is strictly reliant on the integrity of the seals.
Any breach in the vacuum or gas flow system will introduce oxygen. Because the process operates at 1500°C, even trace amounts of oxygen will result in immediate and often irreversible contamination of the Ta4AlC3 phase.
Thermal Lag
Industrial tube furnaces generally have significant thermal mass.
This provides stability but can result in slower cooling rates. While beneficial for annealing, precise programming is required to ensure the material does not spend too much time in intermediate temperature zones where unwanted secondary phases might form.
Making the Right Choice for Your Goal
To maximize the quality of your Ta4AlC3 synthesis, prioritize your parameters based on the specific outcome you need:
- If your primary focus is Phase Purity: Prioritize the integrity of the argon purge cycle to ensure the chamber is completely oxygen-free before the temperature ramps up, protecting the aluminum content.
- If your primary focus is Reaction Completeness: Ensure the furnace can hold the 1500°C temperature consistently without fluctuation to allow full diffusion of the tantalum into the carbon/aluminum matrix.
The success of Ta4AlC3 synthesis lies in the rigorous exclusion of oxygen combined with precise thermal energy management.
Summary Table:
| Feature | Synthesis Requirement | Industrial Tube Furnace Function |
|---|---|---|
| Temperature | Stable 1500°C | Activates atomic diffusion for solid-phase reactions |
| Atmosphere | Inert (High-purity Argon) | Prevents oxidation of reactive Tantalum and Aluminum |
| Thermal Profile | High Uniformity | Ensures consistent phase composition & prevents cold spots |
| Reaction Type | Solid-Phase Synthesis | Acts as a controlled reactor to maintain stoichiometry |
Optimize Your MAX Phase Synthesis with KINTEK Precision
Achieving the perfect Ta4AlC3 phase requires rigorous atmospheric integrity and precise thermal management. Backed by expert R&D and manufacturing, KINTEK offers high-performance Muffle, Tube, Rotary, Vacuum, and CVD systems designed to meet the most demanding lab and industrial requirements. Our furnaces provide the stable 1500°C environments and advanced gas control needed to prevent oxidation and ensure material purity.
Whether you need a standard setup or a system customized for your unique research needs, KINTEK delivers the reliability your materials science requires.
Ready to elevate your material synthesis? Contact KINTEK today to discuss your furnace requirements!
Visual Guide
References
- Mingfeng Li, Yanan Ma. Recent Advances in Tantalum Carbide MXenes: Synthesis, Structure, Properties, and Novel Applications. DOI: 10.3390/cryst15060558
This article is also based on technical information from Kintek Furnace Knowledge Base .
Related Products
- 1700℃ High Temperature Laboratory Tube Furnace with Quartz or Alumina Tube
- 1400℃ High Temperature Laboratory Tube Furnace with Quartz and Alumina Tube
- 1200℃ Split Tube Furnace Laboratory Quartz Tube Furnace with Quartz Tube
- 1800℃ High Temperature Muffle Oven Furnace for Laboratory
- 1700℃ High Temperature Muffle Oven Furnace for Laboratory
People Also Ask
- What special features does the quartz tube furnace have for sample handling? Unlock Visibility and Purity in High-Temp Processes
- What is a high temperature tube furnace? Achieve Precise Heat and Atmosphere Control
- What role does a tube furnace play in the growth of epitaxial thin films via PAD? Essential Guide to Precision Growth
- What is the difference between a tube furnace and a box furnace? Choose the Right Tool for Your Lab
- What design aspects of a split tube furnace influence its performance? Optimize for Temperature Uniformity and Efficiency
- How are tubular furnaces utilized in semiconductor manufacturing? Precision Thermal Processing for High-Yield ICs
- What is the function of a tube furnace for bond-coated substrates? Ensure TBC Durability with Controlled Pre-Oxidation
- What are the limitations of tube furnaces when cracking heavy materials? Overcome Coking and Boost Efficiency



















